Guardians of the Ring—Researchers reveal structure of protein involved in inflammatory diseases

When the body detects a threat, be it a viral invader or an Alzheimer's disease plaque, guardian proteins on the cell surface kick into gear.
The proteins, called gasdermins, set off a cascade of responses that induce cell death and recruit immune cells to the site of perceived danger.
This inflammatory response plays a critical role in health and survival, but, gone awry, it can also inflict harm—from chronic inflammation that contributes to cardiovascular diseases and diabetes to the life-threatening condition known as sepsis, a runaway reaction to infection.
Curbing these haywire immune responses has been challenging, in part because researchers don't yet understand the structures and functions of all the key proteins involved—including gasdermins.
"Hundreds of clinical trials for sepsis have failed," pointed out Hao Wu, the Asa and Patricia Springer Professor of Structural Biology at Harvard Medical School and Boston Children's Hospital. "Gasdermins provide another key point in the disease process where researchers can try to intervene."
Now, Wu and colleagues have captured the first images of a gasdermin down to the atomic level, providing insight into exactly what these proteins look like and how they do their job.
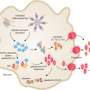
Reporting April 26 in Nature, the researchers found that the most important part of a gasdermin consists of 26 to 28 identical comma-shaped pieces linked together in a ring.
The team also revealed how the pieces morph from a dormant configuration into the ring shape when the gasdermin is called into action.
The findings promise to help researchers design drugs that act on gasdermins, a valuable opportunity since the proteins are involved in "almost every disease of your body related to inflammation," said Wu, senior investigator of the study.
In addition, from a basic-science perspective, "it's just cool to know how the gasdermin structure changes," said Wu.
There are six known types of gasdermins found in various combinations across vertebrate organisms. Wu and colleagues studied gasdermin D from human cells and gasdermin A from mice. They believe their findings will apply to other gasdermin family members in humans, although further studies will need to confirm this.
The team used electron cryomicroscopy, or cryo-EM, to snap high-resolution pictures of the gasdermins.
Specifically, they looked at the gasdermin pore. This is the critical structure gasdermins assemble to "poke a hole in the cell membrane," opening a passage for immune chemicals called cytokines to swarm out of the cell and sound the alarm, said Jianbin Ruan, research fellow in the Wu lab and first author of the study.
The pore also destabilizes the membrane, coaxing the cell to self-destruct to protect against further contamination.
Wu and team found that gasdermin pores displayed beautiful symmetry. Most had 27 repeating units, although some contained 26 or 28 for reasons still unknown.
The researchers were taken aback by how big the pores were. "They're huge," said Wu—although "huge" is a relative term on a cellular scale; the ring is about 30 nanometers wide and the hole it opens is about 20 nanometers wide. That leaves plenty of room for cytokines, typically 4 nanometers wide, to pass through.
Capturing images of the pore at different stages taught the researchers more about how it transforms into its active state. They found that the repeating units stretch from a more compact configuration into their final comma shapes as they bind to the cell membrane and prepare to open the portal.
Pathogens such as bacteria and viruses are known to trigger pore formation. So are substances made by the body, such as cardiovascular or Alzheimer's disease plaques or the uric acid crystals that cause painful episodes of gout.
In addition, cancer cells can either drive gasdermins into action or stop their killing function, said Shiyu Xia, a Ph.D. student in the Wu lab and coauthor of the study.
Although the cytokines released through gasdermin pores recruit much-needed immune cells to fight infections, they also can incite an overreaction or "cytokine storm" that causes massive damage. Wu and colleagues hope the knowledge they've gained will provide new avenues for trying to prevent these sometimes-deadly complications.
More information: Jianbin Ruan et al. Cryo-EM structure of the gasdermin A3 membrane pore, Nature (2018). DOI: 10.1038/s41586-018-0058-6
Journal information: Nature
Provided by Harvard Medical School