New CRISPR technology 'knocks out' yeast genes with single-point precision

The CRISPR-Cas9 system has given researchers the power to precisely edit selected genes. Now, researchers have used it to develop a technology that can target any gene in the yeast Saccharomyces cerevisiae and turn it off by deleting single letters from its DNA sequence.
Such genome-scale engineering - in contrast to traditional strategies that only target a single gene or a limited number of genes - allows researchers to study the role of each gene individually, as well as in combination with other genes. It also could be useful for industry, where S. cerevisiae is widely used to produce ethanol, industrial chemicals, lubricants, pharmaceuticals and more.
Understanding and optimizing the genome could create yeast strains with increased productivity, said study leader Huimin Zhao, a University of Illinois professor of chemical and biomolecular engineering and a member of the Carl R. Woese Institute for Genomic Biology at the U. of I. Zhao's group published the new findings in the journal Nature Biotechnology.
"We want to use microorganisms as cellular factories to make valuable chemicals and biofuels," Zhao said. "The scale we have demonstrated in this study is unprecedented. CRISPR has been used to introduce point mutations - for example, to address genetic diseases - but Saccharomyces yeast has about 6,000 genes, and we want to be able to knock out each of these genes iteratively and find out how they affect the production of a target compound."
Researchers produce "knockout" yeast - where one gene has been deleted, or "knocked out" - to study how each gene contributes to the function of the cell. When a beneficial mutation is found, they can selectively breed yeast with that characteristic. Leading methods to produce knockout yeast excise the entirety of the targeted gene. This creates unintended problems, Zhao said, because many genes overlap each other. Deleting one gene also deletes portions of others, affecting multiple functions and making it difficult for researchers to truly isolate the effects of a single gene.
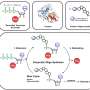
Each letter in a DNA sequence corresponds to a base, the building blocks that make up DNA chains. Zhao's group harnessed the precision of the CRISPR-Cas9 system to create a technique that allows them to delete just one base in a gene's DNA sequence. Since a cell "reads" DNA three bases at a time, this shifts the reading frame and knocks out the gene. Genes that overlap with the edited one remain unchanged and functional.
"We can introduce just one single base change on the entire chromosome. That makes a minimal disturbance in the function of the neighboring genes, so we can study how important the gene is in its cellular context. That kind of precision has not been achieved before," Zhao said.
Their technique, named CRISPR/Cas9 and homology-directed-repair assisted genome-scale engineering or CHAnGE, has the advantages of being quick, efficient and low-cost, in addition to its precision. Zhao's group developed a library of knockout yeast, one for each gene in the S. cerevisiae genome, and are making it available to other researchers for a $50 handling fee.
"In the past, teams of people would spend several years trying to knock out every gene in a yeast. With CHAnGE, one person can generate a library of yeast mutants covering the entire genome in about a month," Zhao said.
Zhao's group is working to develop libraries for other types of yeast, including ones that produce lipids used in lubricants, biofuels and other industrial applications.
More information: Genome-scale engineering of Saccharomyces cerevisiae with single-nucleotide precision, Nature Biotechnology (2018). nature.com/articles/doi:10.1038/nbt4132
Journal information: Nature Biotechnology
Provided by University of Illinois at Urbana-Champaign