First-of-its-kind look at the inner workings of biological protein complexes
Proteins play a crucial role in the human body. These complex molecules help maintain the structure and function of organs and tissue, regulating cellular activity at the most fundamental level.
Understanding the way these proteins work could be the key to using them in the fight against disease – an idea chemists at the University of South Florida have turned into progress.
A new study, led by USF Assistant Professor of Chemistry Ioannis Gelis, Ph.D., published in Nature Communications, is giving researchers a never-before-seen look into the inner workings of the Hsp90-Cdc37-kinase protein complex.
A protein complex is a group of individual protein molecules that come together and interact with one another. Nearly every cellular process requires these complexes to form in order to function properly. However, in many cases, particularly in the presence of cell mutations like in cancer patients, these complexes can further promote progression of the disease.
"If we manage to understand how this molecular machinery works, we hope we can then develop new strategies to inhibit these proteins," said Gelis.
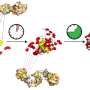
In the protein complex studied by the USF research team, Hsp90 and Cdc37 act as the chaperone or helper proteins, meaning their job is to activate and protect the "client" protein, in this case, a kinase. Once activated, the kinase then facilitates a variety of other cellular functions. In healthy people, this is good. But, for cancer cells which carry mutated kinases, for example, proper functioning of this complex is bad as it promotes cancer progression. In essence, it protects cancer cells.
Gelis says the idea is to stop these cancer cells from functioning the way they typically would. The trick is to prevent the chaperone proteins from activating and protecting the client, allowing the cell's existing defense mechanism to naturally degrade the mutated protein.
"There are many companies and academic groups that are actively working to develop inhibitors that would disrupt these complexes," said Gelis. "Our work shows them how this complex disrupts under natural physiological conditions."
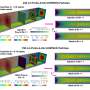
Using nuclear magnetic resonance (NMR) spectroscopy, Gelis and his team were able to obtain high-resolution, three-dimensional images of these proteins. The researchers observed that the addition of only few atoms to Cdc37 (phosphorylation), is a modification that results in a significant structural change of the protein. Once the initial phosphorylation occurs, Hsp90 experiences its own modification which prompts the entire complex to break apart.
"It's a very intricate and intriguing mechanism by which disassociation occurs in these complexes," said Gelis. "The chemical modification in that singular protein is very minor but it brings a huge conformational change that impacts the entire complex. The molecular details revealed in our study contributes toward a more comprehensive understanding of the function of these proteins and will aid in designing better drugs for the treatment of cancer."
More information: Ashleigh B. Bachman et al. Phosphorylation induced cochaperone unfolding promotes kinase recruitment and client class-specific Hsp90 phosphorylation, Nature Communications (2018). DOI: 10.1038/s41467-017-02711-w
Journal information: Nature Communications
Provided by University of South Florida