Computer modeling of WNK kinase inhibitors could offer new tools for understanding hypertension
Researchers from North Carolina State University have modeled and analyzed the binding modes of 210 molecules previously reported to inhibit the function of a family of enzymes involved in regulating salt and blood pressure in the human body. Their findings could help researchers better understand the complex relationships between salt regulation, hypertension and high blood pressure.
The With-No-Lysine (WNK) family of enzymes is a group of four proteins that are involved with blood pressure and body fluid regulation. These enzymes are linked with a rare and severe form of hypertension; however, their individual functions are not well understood. To better comprehend the role of each enzyme, researchers need to develop targeted molecules that can selectively shut off their function. The molecules currently in use bind with all four of the WNK proteins, preventing researchers from teasing out differences in function between each one.
Denis Fourches, assistant professor of computational chemistry, and postdoctoral researcher Melaine Kuenemann used computer modeling to characterize, analyze and visualize each of the 210 molecules known to bind with the WNK family.
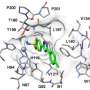
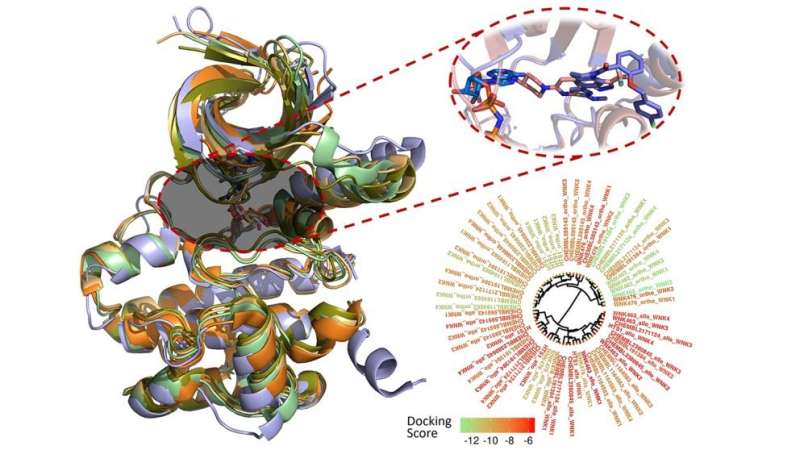
Fourches and Kuenemann created 3-D models of all 210 compounds and docked them into the binding pocket of each WNK enzyme. Then, they measured how well the compounds actually bound and analyzed their specific interactions within the four different binding pockets. They even conducted real-time molecular dynamics simulations to study how these interactions varied over time, revealing new ways to make molecules more selective toward a specific WNK enzyme.
"There aren't that many compounds known to inhibit the WNK kinase family, and the ones we do have bind to all four enzymes," Fourches says. "If we want to better understand the connections between these proteins and hypertension, we need to identify chemicals that can shut down one WNK kinase at a time and thus better interrogate their individual function.
"This is the first study that tries to look at the 'big picture' for these WNK inhibitors using state-of-the-art computer simulations. We hope that our findings will aid medicinal chemists and other researchers in designing new molecules with higher binding potency and selectivity toward each individual WNK kinase."
The work appears in Molecular Informatics.
More information: Melaine A. Kuenemann et al. Cheminformatics Analysis of Dynamic WNK-Inhibitor Interactions, Molecular Informatics (2018). DOI: 10.1002/minf.201700138
Provided by North Carolina State University