Researchers convert CO to CO2 with a single metal atom

Researchers from Washington State University and Tufts University have demonstrated for the first time that a single metal atom can act as a catalyst in converting carbon monoxide into carbon dioxide, a chemical reaction that is commonly used in catalytic converters to remove harmful gases from car exhaust.
The research, published today in the journal Nature Catalysis, could improve catalytic converter design and also has major implications in the field of computational catalysis.
Overcoming lower engine temperatures
As engines have become more efficient, their combustion temperature has become lower, making it harder for catalytic converters to work and creating, paradoxically, more harmful emissions. Car companies have struggled to meet strict emissions standards that aim to protect human health. Volkswagen was even found guilty of having developed a software workaround to cheat on emissions testing.
While studying low-temperature catalysts, the researchers, led by Jean-Sabin McEwen, assistant professor in WSU's Voiland School of Chemical Engineering and Bioengineering, and Charles Sykes, a professor of chemistry at Tufts University, got interested in single metal atoms and their ability to act as catalysts at lower temperatures.
"Most of the harmful chemicals in your exhaust such as carbon monoxide and nitrogen oxide are emitted when starting up the engine," said McEwen. "The lower the temperature, the harder it is to neutralize these harmful chemicals."
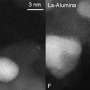
Carbon monoxide to carbon dioxide
In their paper, the researchers demonstrated that the reaction can work with single platinum atoms on a copper oxide support near room temperature. The single platinum atom holds the carbon monoxide in place while the copper oxide supplies the oxygen to convert it into carbon dioxide.
"This is a benchmark study that can guide the design of the next generation of low temperature catalytic converters," said Sykes.

Since catalytic converters use rare and expensive metals like platinum, reducing the use of those elements down to the single atom level could also reduce costs, he added.
Their research also conclusively answers a longstanding debate in the scientific world on whether a single metal atom could act as a catalyst for the oxidation of carbon monoxide to carbon dioxide at low temperatures or whether such a reaction requires a cluster of atoms.
More information: Andrew J. Therrien et al, An atomic-scale view of single-site Pt catalysis for low-temperature CO oxidation, Nature Catalysis (2018). DOI: 10.1038/s41929-018-0028-2
Journal information: Nature Catalysis
Provided by Washington State University