Mechanism behind platinum catalyst captured
Cars are equipped with catalysts to disarm toxic exhaust gases. Platinum plays an important role there. Leiden physicists and chemists have now for the first time seen the mechanism behind a platinum catalyst. With a fundamental understanding of the process, scientists can use this rare material more efficiently. Publication in Nature Communications.
The exhaust gases of over one billion cars worldwide contribute significantly to global warming. But without catalysts, cars would be even more polluting. After toxic exhaust gases leave the engine, catalysts convert those into less harmful substances. Platinum plays an important role here, by eliminating the toxic carbon monoxide. This noble metal is very rare and therefore scientists are researching how to use it as efficient as possible.
Platinum
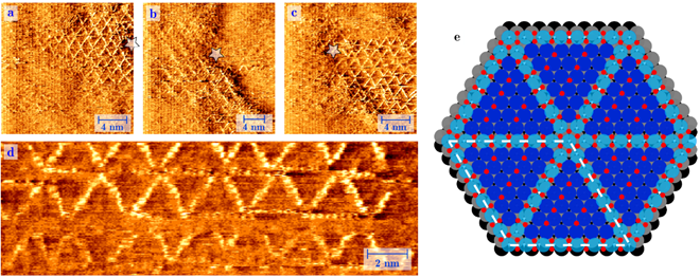
Platinum works as a catalyst by collecting oxygen atoms (O), and letting them bind with the toxic carbon monoxide (CO), to create the less harmful carbon dioxide (CO2). Physicist Joost Frenken and chemists Irene Groot and Matthijs van Spronsen of Leiden University have now for the first time imaged how this process works at the atomic level. With a special home-built microscope they saw an ultra-thin oxygen layer grow on a platinum surface. This happened under realistic circumstances, meaning at the same high pressure and temperature as inside an engine, which made the experiment extra difficult. The researchers discovered that the oxygen atoms are somewhat "loose," so that they can easily react with other substances. This provides for the first time a good explanation for the high catalytic activity of platinum in oxidation reactions.
Efficiency
By unravelling the mechanism behind the platinum catalyst, the Leiden scientists contribute to a better fundamental understanding of catalysis. In the long run, scientists could exploit this knowledge to use rare materials like platinum more efficiently. Groot: "Then we either need less platinum to get the same result, or we understand the catalysis mechanism behind platinum so well that we can create a substitute material."
More information: Matthijs A. van Spronsen et al. Observing the oxidation of platinum, Nature Communications (2017). DOI: 10.1038/s41467-017-00643-z
Journal information: Nature Communications
Provided by Leiden University