Synchrotron study reveals oxygen's influence on the chemistry of atmospheric pollution
Chemical reactions that produce pollutants in the atmosphere, and the chemistry of fuel combustion inside a vehicle engine, have some striking similarities. For each set of reactions, oxygen's role is key. Studying oxygen's part in combustion and atmospheric chemistry could help scientists improve both engines and reduce air pollution, KAUST researchers have shown.
Volatile organic compounds (VOCs) are gaseous molecules that are emitted into the air from the tail pipes and smoke stacks of vehicles, factories and power plants, as well as from living plants. VOCs undergo a sequence of auto-oxidation reactions with oxygen from the surrounding air to form highly oxygenated molecules that contribute to air pollution and produce aerosols that are known to affect the climate.
Auto-oxidation also occurs during the ignition and combustion of fuels. But revealing the identity of the molecules from these reactions has been difficult, say Zhandong Wang and Mani Sarathy from the Clean Combustion Research Center, who co-led the work. "The highly oxygenated intermediates produced from auto-oxidation are very reactive and decompose quickly," Wang says.
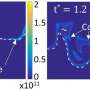
So Wang, Sarathy and their team developed an advanced experimental setup to sample these elusive molecules before they decompose. "We used a sophisticated technique—a jet-stirred reactor coupled with synchrotron radiation photoionization and molecular-beam mass spectrometry—at the Advanced Light Source in Berkeley," says Wang. The team also used a high-resolution atmospheric-pressure chemical-ionization mass spectrometer at the Analytical Core Laboratory at KAUST to analyze combustion auto-oxidation products.
Current theoretical models of combustion chemistry assume one, or possibly two, oxygen molecules can attach to a fuel molecule during auto-oxidation. Wang and Sarathy's results show that at least three sequential oxygen-addition reactions, and possibly more, can take place. "Our most significant finding is that auto-oxidation processes leading to auto-ignition are much more complex than previously thought," says Wang. "We have shown that many large hydrocarbon and oxygenated fuels exhibit extensive auto-oxidation, and when these pathways are included in models, they significantly alter simulation results."
Updating these models will allow the team to more accurately simulate fuel combustion and to potentially improve the performance of real engines. But the findings are broader reaching. "We are working with the atmospheric scientists from University of Helsinki to further explore analogous auto-oxidation processes in atmosphere and combustion. Our goal is to use our combustion experience to develop models for atmospheric aerosol formation via VOC auto-oxidation. This could significantly improve simulations to predict air pollution and global temperature."
More information: Zhandong Wang et al. Unraveling the structure and chemical mechanisms of highly oxygenated intermediates in oxidation of organic compounds, Proceedings of the National Academy of Sciences (2017). DOI: 10.1073/pnas.1707564114
Journal information: Proceedings of the National Academy of Sciences