Researchers develop potentially low-cost, low-emissions technology that can convert methane without forming CO2
As we work to toward more sustainable ways of powering our lifestyles, there is a quest to bridge the gap between the carbon dioxide-emitting fossil fuels we rely on for our most basic needs, and the cleaner, but not yet economically feasible alternative technologies.
To that end, a group at UC Santa Barbara has explored methods by which currently cheap and abundant methane (CH4) can be reduced to clean-burning hydrogen (H2) while also preventing the formation of carbon dioxide (CO2), a greenhouse gas. Its report, "Catalytic molten metals for the direct conversion of methane to hydrogen and separable carbon," appears in the journal Science.
"In the U.S., methane will be the heart of our economy for four or five decades, and figuring out ways to use it more sustainably is what motivates us," said UCSB chemical engineering professor Eric McFarland. "This paper was an interesting angle on something we've been looking at for a long time."
A product of both natural and man-made processes, methane—the primary component of natural gas—is an important source of fuel for cooking, heating and powering our homes and is used in manufacturing and transportation. As a waste product that is a more potent greenhouse gas than carbon dioxide, it is the target of many efforts to capture and reduce such emissions.
Steam methane reforming (SMR) has been commercialized for decades and is the most common process for producing commercial hydrogen. However, the researchers point out, SMR consumes significant amounts of energy and necessarily produces carbon dioxide, which is usually released into the atmosphere. When the process was introduced, CO2 was not considered a problem. But as we became more greenhouse gas-conscious, it has grown into a global concern. The cost of operating the SMR process, and the potential additional costs of carbon taxes and carbon sequestration, puts hydrogen production by SMR at risk for significant cost increases—especially in smaller scale operations that might provide the hydrogen needed for fuel cell vehicles.
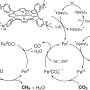
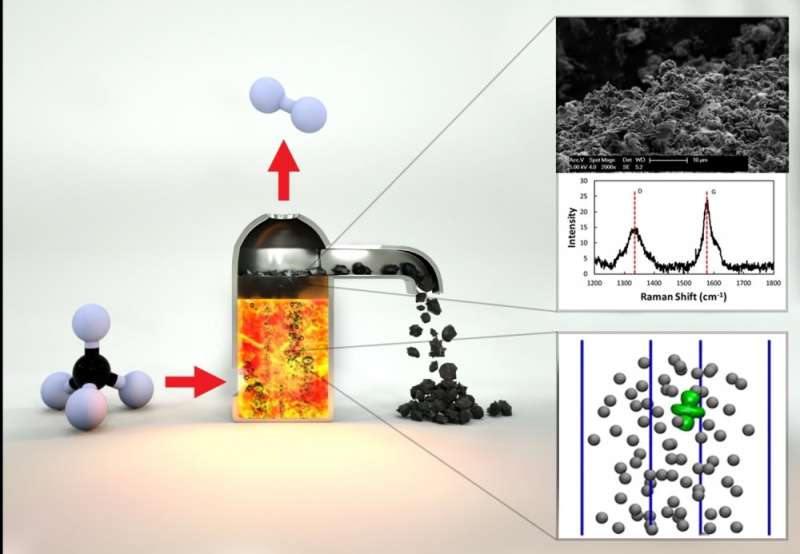
The UCSB team includes a longstanding collaboration on catalytic approaches to natural gas conversion between theoretical chemist and professor Horia Metiu and McFarland. Together with chemical engineering professor Michael Gordon, they began investigating the use of molten metals and molten salts as interesting and unexplored catalytic systems. Metiu's theoretical work suggested that different combinations of metals in molten alloys might provide increased catalytic activity for converting methane into hydrogen and solid carbon. The researchers have developed a single-step method by which methane can be converted into hydrogen, which is not only simpler and potentially less expensive than conventional SMR methods, and results in a solid form of carbon that can be readily transported and stored indefinitely.
"You introduce a bubble of methane gas into the bottom of a reactor filled with this catalytically active molten metal," McFarland explained. "As the bubble rises, the methane molecules hit the wall of the bubble and they react to form carbon and hydrogen."
Eventually, he continued, by the time the methane bubble reaches the surface, it has broken down into hydrogen gas, which is released at the top of the reactor; carbon solids that float to the top of the liquid metal can then be skimmed off. Compared to conventional methods that rely on reactions that occur on solid surfaces, the molten metal alloy surfaces are not deactivated by the accumulation of carbon and can be reused indefinitely. The combination of an active liquid metal and its solubility to hydrogen allows the melt to take up relatively more hydrogen and carbon than may be present in the gas bubbles. This allows the process to be efficient with very high-pressure methane to produce high-pressure hydrogen.
"You're really allowing yourself to pull all the products away from the reactants and that causes the equilibrium to be shifted toward the products. The process in principle can operate at high pressure and still get very high methane conversion," McFarland said.
The ecosystem for deploying this type of technology already exists, given existing infrastructure for processing hydrocarbons such as coal and natural gas, the current abundance of methane, and legislative and industry efforts to tighten up the capture of fugitive emissions, according to McFarland. The research has captured the attention and support of Royal Dutch Shell, he added. The electricity produced from hydrogen derived by this zero-carbon dioxide process would be cheaper than current rates for solar energy, which, while ultimately more sustainable, is not cost competitive with fossil fuels today.
"If the entire world is wealthy, then wind and solar would be sufficiently low cost to be widely deployed, but it's not cheap enough for the world that we have today," McFarland said. From an emissions standpoint, he continued, it is particularly important to deploy low-cost, low-emissions technologies in places such as China, currently the world's largest emitter of greenhouse gases. India and Africa, which have enormous and growing hydrocarbon consumptions, would benefit from such technology also; they are not rich enough yet to have the luxury of solar panels.
More information: D. Chester Upham et al. Catalytic molten metals for the direct conversion of methane to hydrogen and separable carbon, Science (2017). DOI: 10.1126/science.aao5023
Journal information: Science
Provided by University of California - Santa Barbara