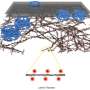
Super-resolution microscopy reveals lamin protein meshwork at the inner side of the nuclear membrane
All creatures of the animal kingdom share one thing: the nuclear membrane. Wrapping the genetic core of the cell, this membrane, together with all the attached proteins, plays a vital role in biological functions. Despite its importance, details of its architecture are still missing.
Scientists at the A*STAR Institute of Medical Biology, led by Brian Burke, have constructed a nanoscale model of the inner side of the mammalian nuclear membrane, where threadlike proteins called lamins form a mesh. Lamins, as well as providing support for the nuclear membrane, are involved in cell division, chromatin organization and DNA repair and more. Mutations in lamins have been connected to more than a dozen human diseases, including muscular dystrophy, heart disease and progeria, a premature aging syndrome.
Because the nuclear periphery is so crowded with protein and DNA molecules, it has proven extremely difficult to determine the lamin arrangement using either light or electron microscopy. Now Burke's team has used super-resolution microscopy to go beyond the capabilities of conventional microscopy and to record the locations of single lamin molecules.
Burke explains the process. "Fusing 10,000 images, each fluorescently-labeled lamin appeared as a bright spot. Then, we applied a mathematical technique to link these spots. As a result we saw that irregular filament networks made of different types of lamins cover the entire nuclear inner surface," he says. "Significantly, A-type and B-type lamins associate only with other lamins of the same type, forming independent networks."
Although the different lamins are almost identical, they interact with distinct proteins and therefore seem to have different functions. For example, the A-type lamin, LaC, binds to a protein that is part of the channels surrounding the holes on the nuclear membrane. The nuclear membrane has pores that allow the transfer of material between the nucleus and the rest of the cell. These channels are known as nuclear pore complexes (NPCs) and look like hoops with a basketball net. The interaction between LaC and a protein in the basket could mean that LaC filaments regulate the distribution of the NPCs in the nuclear membrane, a mechanism which may be linked to the development of progeria.
Burke says the next challenges for the team are to better understand the different functions of the lamin networks and to explore their interactions with other nuclear components. Their findings may explain how even subtle changes in nuclear lamin organization can give rise to a bewildering array of human diseases.
More information: Wei Xie et al. A-type Lamins Form Distinct Filamentous Networks with Differential Nuclear Pore Complex Associations, Current Biology (2016). DOI: 10.1016/j.cub.2016.07.049
Journal information: Current Biology