Protein derived from oats is tethered to 'cell-suicide' enzyme in new technique

The ability to selectively eradicate specific types of cells from multicellular organisms allows scientists to decipher those cells' functions, but the tools available to do so—whether surgical, chemical, or genetic—are imprecise and far from ideal. Now, protein engineers and neurobiologists at UC San Francisco have teamed up to create a biological light saber—an engineered protein that can slay specific cells simply by exposing them to light.
Such cell ablation techniques have been particularly important in neuroscience. Some of the earliest insights on brain function were based on observations of patients who had specific lesions in different brain areas. Scientists subsequently developed precise methods to emulate such "experiments in nature," ablating particular cells or tissues in order to dissect their normal function. Most recently, researchers have used laser ablations, genetically triggered toxins, and even light-based switches that set oxidizing agents off on a killing spree. But these techniques lack precision and usually create more damage, including from inflammation, than is necessary.
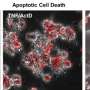
The new study—to be published during the week of September 11, 2017 in the Online Early Edition of Proceedings of the National Academy of Sciences (PNAS)—reports that the protein, which the scientists dubbed "Caspase-LOV," uses light to leverage apoptosis, a natural "cell-suicide" pathway by which cells can trigger their own death under certain circumstances.
Caspase-LOV, devised in experiments led by graduate student Ashley Smart, is so called because it links LOV (light-oxygen-voltage-sensing domain), a molecular light sensor derived from oats, to caspase-3, the human version of an enzyme that is crucial to apoptosis.
Because apoptosis is a fundamental biological process, employed during normal development to prune unneeded cells, and to dispose of defective ones, it is razor-sharp and induces a clean, non-inflammatory form of cell death. When cells are acutely injured, they spill all their contents, but apoptotic cells shrink and are absorbed by neighboring cells or by immune cells—the caspase endgame is a tidy cell death without any inflammation of surrounding tissue.
"Caspases are like demolition experts," said James A. Wells, PhD, professor and chair of pharmaceutical chemistry at the UCSF School of Pharmacy, and co-senior author of the new study "They know where to put explosives in a bridge to bring it down without having to use a nuclear weapon. One of the reasons for using caspases to kill is because they do so in a very clean way—unlike ablating cells with lasers or surgery, which is messy and not precise, this leads to a corpse-removal process which nature knows how to deal with, without any collateral damage to the tissue."
Smart and Wells were joined in the study by co-senior author Graeme "Grae" Davis, PhD, the Albert Bowers Professor and chair of the Department of Biochemistry and Biophysics at UCSF, and a member of the UCSF Kavli Institute for Fundamental Neuroscience.
Since light easily penetrates the embryos of the fruit fly Drosophila, the authors believe Caspase-LOV will be immediately useful to labs, such as Davis's, that study this important model organism, but they said the tool could also eventually be used in other organisms, such as mice, by using fiber optics to illuminate and precisely eliminate cells.
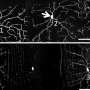
The Caspase-LOV cell-death switch works simply and elegantly. Scientists can decide in advance which cell types will carry Caspase-LOV, and also control when light strikes those cells to induce apoptosis. The team demonstrated in their new study, for example, that when the Caspase-LOV was expressed in fruit-fly motor neurons, those neurons died upon exposure to light, thus affecting the flies' ability to move. Similarly, the team was able to selectively ablate specific cells in the retina simply by exposing the fruit fly's compound eyes to light.
Because the duration of light exposure is directly correlated with the extent of cell death, Wells said, Caspase-LOV could be a powerful tool to explore the mechanisms of neurodegeneration, a process that occurs over an extended period of time in human diseases such as Alzheimer's and Parkinson's.
"We're excited about this," said Wells, also the Harry Wm. and Diana V. Hind Distinguished Professor of Pharmaceutical Sciences at UCSF. "There are already a couple of labs using it, and I think more will follow now that we've published these findings."
More information: Ashley D. Smart el al., "Engineering a light-activated caspase-3 for precise ablation of neurons in vivo," PNAS (2017). www.pnas.org/cgi/doi/10.1073/pnas.1705064114
Journal information: Proceedings of the National Academy of Sciences
Provided by University of California, San Francisco