Oil and water may mix under extreme pressure
They say that oil and water do not mix … but now scientists have discovered that – under certain circumstances – it may be possible.
A new study suggests that some oily molecules – which normally repel water – can be forced to dissolve in water when the two substances are squeezed together under extreme pressure. Researchers at Edinburgh applied high pressure to tiny containers filled with water and methane, creating conditions similar to the intense pressure found on the ocean floor or inside the planets Uranus and Neptune.
Water-repelling substances
By compressing water and methane together, scientists have been able to gain insights into how the chemicals interact. Methane is often used in experiments to study the properties of substances like oil that repel water – called hydrophobic molecules. The new findings suggest it may be possible to mix other hydrophobic molecules with water in a similar way.
The study is published in the journal Science Advances.
The team squeezed methane and water molecules between two ultra-sharp diamonds and compressed them by bringing the two anvil points together. The diamond anvil was used to apply pressures of up to 20,000 Bars – 20 times greater than the pressure at the bottom of the Mariana trench, the deepest part of the world's oceans.
Compacted molecules
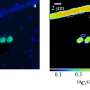
Under a microscope, methane – much like oil – appears as large droplets in water at normal pressure, demonstrating that the substances do not mix. However, the team found the droplets disappeared at high pressures, indicating that the methane had dissolved.
Researchers think this happens because methane molecules shrink as pressure is increased, while water molecules stay largely the same. This could allow compacted methane molecules to fit between the much larger water molecules, enabling them to mix, the team says.
Useful applications
Understanding the mixing properties of water and methane could help researchers find ways of replacing expensive and hazardous solvents used in industry.
It could also help provide new insights into conditions at the bottom of the ocean or in the outer solar system. The study was supported by the Engineering and Physical Sciences Research Council and European Research Council.
"These exciting findings shed light on how water-repelling substances behave under high pressures, such as those found at the ocean floor or inside planets. This could have a huge range of applications, from replacing expensive and environmentally hazardous industrial solvents to modelling planetary bodies like Saturn's largest moon, Titan," says Dr John Loveday.
More information: Ciprian G. Pruteanu et al. When immiscible becomes miscible—Methane in water at high pressures, Science Advances (2017). DOI: 10.1126/sciadv.1700240
Journal information: Science Advances
Provided by University of Edinburgh