Mixed valence states in lead perovskites
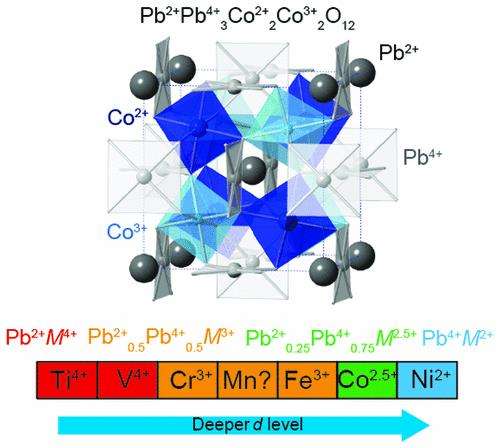
Scientists at Tokyo Institute of Technology, the Kanagawa Academy of Science and Technology have reported an unusual charge distribution of Pb2+Pb4+3Co2+2Co3+2O12 for a perovskite PbCoO3 synthesized at 12 GPa, with charge orderings in the A and B sites of an ABO3 perovskite. This strategy can possibly lead to the production of next-generation materials with fascinating properties such as superconductivity, colossal magnetoresistance, and high thermopower.
Transition metals (TMs) exhibit charge degree of freedom, resulting in interesting properties, such as charge ordering related to metal-insulator transitions, high-temperature superconductivity, colossal magnetoresistance, and high thermopower. Metal ions with half-integer valence tend to split into two spatially ordered integer valence ions. To realize a half-integer valence state and charge ordering in the B site of a perovskite ABO3, two or more elements with different valences need to be mixed in the A site.
A group of researchers, Prof. Masaki Azuma from Tokyo Institute of Technology, and Dr.Yuki Sakai at the Kanagawa Academy of Science and Technology and colleagues, have reported an unusual half-integer average charge system Pb3.5+M2.5+O3 with charge ordering in the A and B sites of a perovskite PbCoO3. Furthermore, the charge orderings in these sites were stabilized by tuning the energy levels of the Pb 6s and TM 3d orbitals. Bond valence sum calculations revealed a valence distribution of Pb2+Pb4+3Co2+2Co3+2O12, with Pb and Co exhibiting charge ordering despite the chemical composition of PbCoO3. As expected, the average oxidation state was Pb3.5+Co2.5+O3, with half-integer valences in both A and B sites of the perovskite structure stabilized by the balanced Pb 6s and Co 3d levels. The valence distribution of PbMO3 was controlled by tuning the depth of the d level of M. The complex valence distribution is expected to change on perturbations, e.g., pressure and chemical modification. For instance, when the Co charge ordering is melted, Pb2+0.25Pb4+0.75Co2.5+O3 is formed, Pb2+0.5Pb4+0.5Co3+O3 is first formed by the intermetallic charge transfer between Pb and Co and then possibly Pb2+Co4+O3 under pressure.
In the future, the application of the strategy of realizing mixed valence states in the A and B sites of perovskite compounds via the tuning of the energy difference between Pb 6s and transition metal 3d orbitals will be reported for other systems with valence-skipping elements, e.g., Au, Tl, and Sb.
More information: Yuki Sakai et al, A-Site and B-Site Charge Orderings in anLevel Controlled Perovskite Oxide PbCoO, Journal of the American Chemical Society (2017). DOI: 10.1021/jacs.7b01851
Journal information: Journal of the American Chemical Society
Provided by Tokyo Institute of Technology




















