Chemical potential effect found to depend on electronic structure of material
The chemical potential is a fundamental concept in condensed matter physics. While the relevant equations which define it can be found in any undergraduate physics textbook, its temperature dependence in systems which are good conductors is usually insignificant. As a result, despite intensive research interest in FeSe, an unconventional superconductor exhibiting several extraordinary properties, the temperature dependence of the chemical potential has been previously overlooked.
In a recent paper published as an Editor's Suggestion in Physical Review B, collaboration between the I05 beamline team at Diamond Light Source and Royal Holloway University of London have shown that, based on the fine details of the electronic structure of the material, a substantial variation of the chemical potential effect is to be expected. They then tested this hypothesis using high-resolution angle-resolved photoemission spectroscopy measurements (ARPES) at the ARPES beamline (I05) at Diamond, finding an even larger effect experimentally than in their theoretical modelling. On the other hand, the shift of the chemical potential is the only observed effect, ruling out an alternatively scenario in which the electronic bands evolve continuously by themselves as a function of temperature. The results have important implications for the understanding of the intricate behaviour of FeSe, particularly at higher temperatures.
The chemical potential – always important, but sometimes overlooked
Electrons in solids obey two basic rules: first they cannot share the same state as another electron, and second they generally like to occupy the lowest available energy states. As a result, electrons 'fill up' all available states starting from the lowest energy states available, one electron per state, up to a level when all the electrons have been counted. Scientists refer to the level which separates the occupied from the unoccupied states as the 'chemical potential'. Things become a bit fuzzy at high temperature, because thermal energy fluctuations allows electrons to briefly occupy a state above the chemical potential according to a well-known probability distribution, but the concept of the chemical potential is still very useful, and pops up all over condensed matter physics (and chemistry too, as the name suggests). In fact the temperature dependence of the chemical potential is an important concept in semiconductor physics, playing a crucial role in determining the temperature dependence of the resistance of the sample, for instance. However in good metals, for example elemental copper, the chemical potential is still an important parameter, but any changes of the chemical potential varies as a function of temperature are usually insignificant.
The unique properties of FeSe
In this study, the researchers focused on an unexpectedly strong temperature dependence of the chemical potential in FeSe. Why FeSe? In brief: it may seem like a simple system with only two elements, with the samples being constructed from layers of square Fe-Se nets, but its fascinating properties have attracted interest from many experimental and theoretical groups worldwide. FeSe has become a test bed for theories which purport to explain the phenomenon of unconventional and high temperature superconductivity in the wider family of iron-based superconductors. While the superconductivity in normal FeSe kicks in only at 8 degrees above absolute zero (8 Kelvin, -265°C), this 'critical temperature' can be raised fourfold by squeezing it very tightly (at 8000 times atmospheric pressure), and is perhaps as high as 100 Kelvin (i.e. 100 degrees above absolute zero, -173°C) when it is grown as a single layer in a particular manner. Back in the normal samples of FeSe, it has also been shown that the superconductivity is strongly and unusually influenced by the fact that the square nets actually slightly distort into rectangles at 90 Kelvin (-183°C).
All of these intriguing physical properties provide an excellent motivation to study the electronic states inside the sample. The technique of choice is angle-resolved photoemission spectroscopy (ARPES); where an intense beam of light (photons) is focused onto a sample, which emits electrons according to the photoelectric effect, as was first understood by Einstein in 1905. By analysing the energy and momentum of the electrons kicked out of the sample in this way, scientists are able to map out the allowed energy and momentum relationship of electrons inside the material. In fact, high resolution measurements of the electronic structure of FeSe by ARPES at beamline I05 at Diamond have previously made several important experimental contributions to the understanding of this material, particularly with regards to the influence of the square-rectangle distortion of the FeSe layers, happening below 90 Kelvin (-183°C). However, in this study the researchers focused on measuring in the square phase only, from 100 Kelvin (-173°C) up to room temperature (300 Kelvin, 27°C).
Large temperature dependence of the chemical potential predicted and observed in FeSe
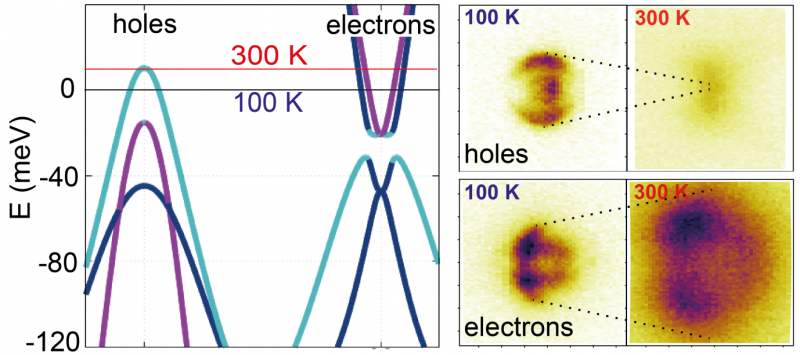
The first step for the researchers was to use the experimental data obtained at 100 Kelvin (-173°C) to construct an accurate theoretical model of the electronic states in the system. This was done using a 'tight-binding model', where one considers electrons sitting on particular Fe sites in the lattice, and then allowing them to 'hop' onto neighbouring sites. By adjusting the parameters of the model, it was possible to achieve a high level of accuracy, compared with the experimental results. They showed that this model predicted a large temperature dependence of the chemical potential.
The reason to expect a large temperature dependence of the chemical potential is that while FeSe is a metal in the sense that it can carry electric currents with a finite resistance (above the superconducting transition temperature), it is far from being a typical metal. In fact it is known that there are two sorts of charge carriers in the system, the 'electron-like' and 'hole-like' carriers. These names come about from the behaviour of electrons in solids: the electrons all interact with one another so they are far from displaying the behaviour of a free electron in a vacuum, but one can often use a description of electrons with a modified 'effective mass', with the terms 'electron-like' and 'hole-like' referring to whether that effective mass is positive (i.e. like a free electron) or negative effective mass (a hole).
In FeSe, the number of 'electrons' and 'holes' is constrained to be equal in order for the system to be neutrally charged overall. Actually one needs to be a bit more precise than this: "the chemical potential at any given temperature will adjust such that the thermally-averaged populations of electrons and holes remain equal" said Luke Rhodes, a joint PhD student between Diamond and Royal Holloway, and the lead author of the study. In FeSe there is a natural asymmetry between the electron and holes; while the electrons have plenty of available states above the chemical potential for electrons to jump up into with thermal fluctuations, there are hardly any available for the holes. As a result of this asymmetry, on top of the fact that the number of electrons and holes is rather small in FeSe, theoretical calculations indicated that raising the temperature would require a substantial adjustment of the chemical potential.
The researchers then turned to high resolution ARPES at the I05 beamline at Diamond in order to experimentally confirm this effect. Using high-quality samples grown at the University of Oxford, they measured the electronic structure of FeSe as a function of temperature from 100 to 300 Kelvin (-173°C to 27°C), which had not been previously studied. They directly observed a significant variation of the chemical potential, which was even larger than in the theoretical calculation.
Implications for modelling FeSe
In order to understand the various intriguing properties of FeSe, theorists often start with models of the electronic structure and then investigate what sort of tendencies and susceptibilities the model has towards different kinds of phase transitions. However, as found in this study, the details of the model really matter. "We have shown that it is important to start with an accurate theoretical model, and we've also shown that the chemical potential must always be carefully taken into account", said Luke Rhodes. The research team now intend to use their model to investigate the square-rectangular phase transition of FeSe at 90 Kelvin (-183°C), where they suspect the chemical potential may also be playing an important role.
More information: L. C. Rhodes et al. Strongly enhanced temperature dependence of the chemical potential in FeSe, Physical Review B (2017). DOI: 10.1103/PhysRevB.95.195111
Journal information: Physical Review B
Provided by Diamond Light Source