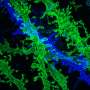
New microscopy method breaks color barrier of optical imaging
Researchers at Columbia University have made a significant step toward breaking the so-called "color barrier" of light microscopy for biological systems, allowing for much more comprehensive, system-wide labeling and imaging of a greater number of biomolecules in living cells and tissues than is currently attainable. The advancement has the potential for many future applications, including helping to guide the development of therapies to treat and cure disease.
In a study published online April 19 in Nature, the team, led by Associate Professor of Chemistry Wei Min, reports the development of a new optical microscopy platform with drastically enhanced detection sensitivity. Additionally, the study details the creation of new molecules that, when paired with the new instrumentation, allow for the simultaneous labeling and imaging of up to 24 specific biomolecules, nearly five times the number of biomolecules that can be imaged at the same time with existing technologies.
"In the era of systems biology, how to simultaneously image a large number of molecular species inside cells with high sensitivity and specificity remains a grand challenge of optical microscopy," Min said. "What makes our work new and unique is that there are two synergistic pieces - instrumentation and molecules - working together to combat this long-standing obstacle. Our platform has the capacity to transform understanding of complex biological systems: the vast human cell map, metabolic pathways, the functions of various structures within the brain, the internal environment of tumors, and macromolecule assembly, to name just a few."
All existing methods of observing a variety of structures in living cells and tissues have their own strengths, but all are also hindered by fundamental limitations, not the least of which is the existence of a "color barrier."
Fluorescence microscopy, for example, is extremely sensitive and, as such, is the most prevalent technique used in biology labs. The microscope allows scientists to monitor cellular processes in living systems by using proteins that are broadly referred to as "fluorescent proteins" with usually up to five colors. Each of the fluorescent proteins has a target structure that it applies a "tag," or color to. The five fluorescent proteins, or colors, typically used to tag these structures are BFP (Blue Fluorescent Protein), ECFP (Cyan Fluorescent Protein), GFP (Green Fluorescent Protein), mVenus (Yellow Fluorescent Protein), and DsRed (Red Fluorescent Protein).
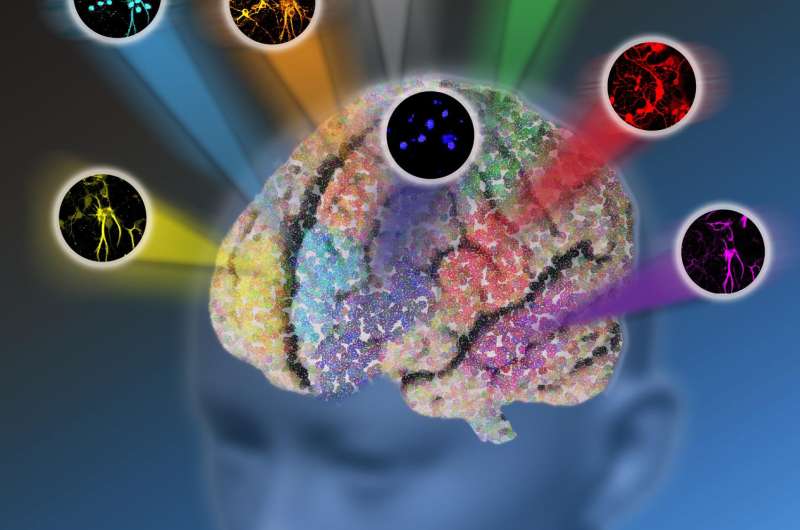
Despite its strengths, fluorescence microscopy is impeded by the "color barrier," which limits researchers to seeing a maximum of only five structures at a time because the fluorescent proteins used emit a range of indistinguishable shades that, as a result, fall into five broad color categories.
If a researcher is trying to observe all of the hundreds of structures and different cell types in a live brain tumor tissue sample, for example, she would be restricted to seeing only up to five structures at a time on a single tissue sample. If she wanted to see more than those five, she would have to clean the tissue of the fluorescent labels she used to identify and tag the last five structures in order to use those same fluorescent labels to identify another set of up to five structures. She would have to repeat this process for every set of up to five structures she wants to see. Not only is observing a maximum of five structures at a time labor intensive, but in cleaning the tissue, vital components of that tissue could be lost or damaged.
"We want to see them all at the same time to see how they're operating on their own and also how they're interacting with each other," said Lu Wei, lead author on the study and a postdoctoral researcher in the Min lab. "There are lots of components in a biological environment and we need to be able to see everything simultaneously to truly understand the processes."
In addition to fluorescence microscopy, there are currently a variety of Raman microscopy techniques in use for observing living cell and tissue structures that work by making visible the vibrations stemming from characteristic chemical bonds in structures. Traditional Raman microscopy produces the highly-defined colors lacking in fluorescence microscopy, but is missing the sensitivity. As such, it requires a strong, concentrated vibrational signal that can only be achieved through the presence of millions of structures with the same chemical bond. If the signal from the chemical bonds is not strong enough, visualizing the associated structure is near impossible.
To address this challenge, Min and his team, including Profs. Virginia Cornish in chemistry and Rafael Yuste in neuroscience, pursued a novel hybrid of existing microscopy techniques.
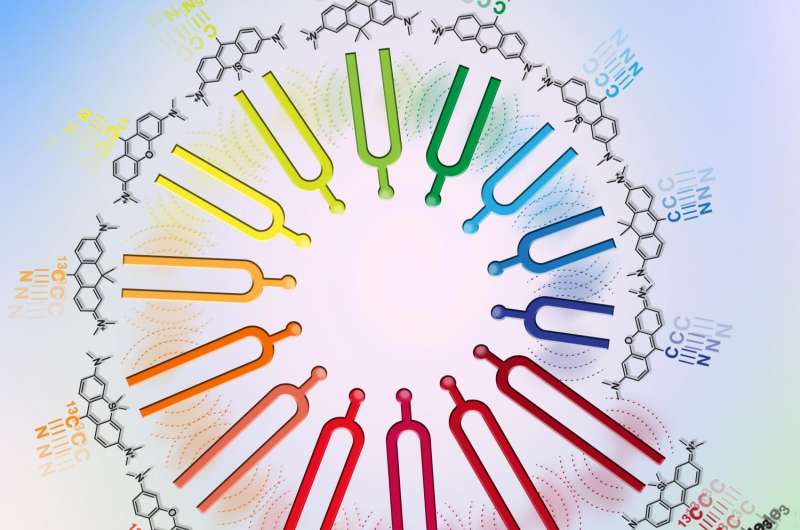
They developed a new platform called electronic pre-resonance stimulated Raman scattering (epr-SRS) microscopy that combines the best of both worlds, bringing together a high level of sensitivity and selectivity. The innovative technique identifies, with extreme specificity, structures with significantly lower concentration - instead of millions of the same structure needed to identify the presence of that structure in traditional Raman microscopy, the new instrument requires only 30 for identification. The technique also utilizes a novel set of tagging molecules designed by the team to work synergistically with the ultramodern technology. The amplified "color palette" of molecules broadens tagging capabilities, allowing for the imaging of up to 24 structures at a time instead of being limited by only five fluorescent colors. The researchers believe there's potential for even further expansion in the future.
The team has successfully tested the epr-SRS platform in brain tissue. "We were able to see the different cells working together," Wei said. "That's the power of a larger color palette. We can now light up all these different structures in brain tissue simultaneously. In the future we hope to watch them function in real time." Brain tissue is not the only thing the researchers envision this technique being used for, she added. "Different cell types have different functions, and scientists usually study only one cell type at a time. With more colors, we can now start to study multiple cells simultaneously to observe how they interact and function both on their own and together in healthy conditions versus in disease states."
The new platform has many potential applications, Min said, adding that it is possible the technique could one day be used in the treatment of tumors that are hard to kill with available drugs. "If we can see how structures are interacting in cancer cells, we can identify ways to target specific structures more precisely," he said. "This platform could be game-changing in the pursuit of understanding anything that has a lot of components."
More information: Lu Wei et al, Super-multiplex vibrational imaging, Nature (2017). DOI: 10.1038/nature22051
Journal information: Nature
Provided by Columbia University