Killing flu viruses with help from a frog

Frog mucus is loaded with molecules that kill bacteria and viruses, and researchers are beginning to investigate it as a potential source for new anti-microbial drugs. One of these "host defense peptides," courtesy of a colorful tennis-ball-sized frog species (Hydrophylax bahuvistara) from southern India, can destroy many strains of human flu and protect mice against flu infection, researchers report April 18 in the journal Immunity.
This peptide is far from becoming an anti-flu drug, but this is the first evidence of its flu-killing ability. It seems to work by binding to a protein that is identical across many influenza strains, and in lab experiments, it was able to neutralize dozens of flu strains, from the 1934 archival viruses up to modern ones. The researchers named the newly identified peptide "urumin," after the urumi, a sword with a flexible blade that snaps and bends like a whip, which comes from the same Indian province, Kerala, as the frog.
"Different frogs make different peptides, depending on where their habitat is. You and I make host defense peptides ourselves," says flu specialist and study co-author Joshy Jacob of Emory University. "It's a natural innate immune mediator that all living organisms maintain. We just happened to find one that the frog makes that just happens to be effective against the H1 influenza type."
Practically all animals make at least a few anti-microbial host defense peptides as part of their innate immune systems, and researchers are only beginning to catalog them. However, frogs have drawn the most attention as a source of host defense peptides, because it's relatively easy to isolate the peptides from their mucus. Researchers can simply give the frogs a small electric shock or rub a powder on the frogs to make them secrete their defense peptides, which can then be collected.

Researchers from the Rajiv Gandhi Center for Biotechnology in Kerala, India, have been isolating peptides from their local frogs and screening them for potential anti-bacterials, but Jacob wondered if there might also be peptides that neutralize human-infecting viruses. Jacob and his colleagues screened 32 frog defense peptides against an influenza strain and found that 4 of them had flu-busting abilities.
"I was almost knocked off my chair," says Jacob. "In the beginning, I thought that when you do drug discovery, you have to go through thousands of drug candidates, even a million, before you get 1 or 2 hits. And here we did 32 peptides, and we had 4 hits."
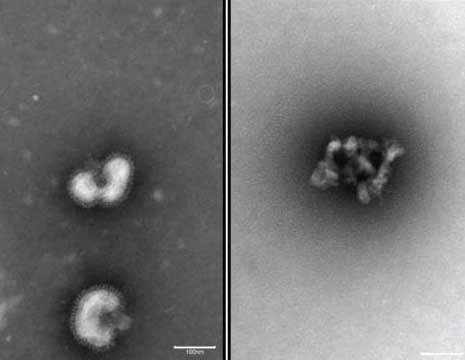
Unfortunately, when the researchers exposed isolated human red blood cells (in a dish) to the flu-buster peptides, three out of the four proved toxic. However, the fourth—urumin—seemed harmless to human cells but lethal to a wide range of flu viruses. Electron microscope images of the virus after exposure to urumin reveal a virus that has been completely dismantled.
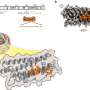
Jacob's team is still working out the details of the flu-destroying mechanism, but the urumin appears to work by targeting a viral surface protein called hemagluttinin, the H in H1N1. "The virus needs this hemagglutinin to get inside our cells," says Jacob. "What this peptide does is it binds to the hemagglutinin and destabilizes the virus. And then it kills the virus."
More information: Immunity, Holthausen et al.: "An Amphibian Host Defense Peptide Is Virucidal for Human H1 Hemagglutinin-Bearing Influenza Virusest" www.cell.com/immunity/fulltext … 1074-7613(17)30128-0 , DOI: 10.1016/j.immuni.2017.03.018
Journal information: Immunity
Provided by Cell Press