A case where smoking helped: Scientists help understand mechanics of rare hemoglobin mutation
There's at least one person in the world for whom smoking has a beneficial effect, and it took an international collaboration of scientists led by a Rice University professor to figure out why.
Rice biochemist John Olson and collaborators in Germany and France helped a young woman and her father understand why she has anemia but her father, who is a smoker, does not.
The woman, who was in her 20s when diagnosed, and her father share a mutation in the gene that encodes hemoglobin, the protein in red blood cells responsible for taking up and delivering oxygen to cells around the body. The mutation is one of more than 1,000 discovered so far in adult human hemoglobin. Most appear to have no effect on people, but when medical problems occur, the disease is called a hemoglobinopathy and often named after the city or hospital where it was discovered. In this case, the family was living in Mannheim, Germany, but the father was born in the Turkish city of Kirklareli.
The Kirklareli mutation did not affect the iron content of her dad's blood, but did appear to be the root cause of the young woman's chronic anemia, according to the researchers. Further investigation revealed that absorbing carbon monoxide from cigarette smoke is therapeutic for those with this rare genetic disorder.
A paper on the research appeared this month in the Journal of Biological Chemistry.
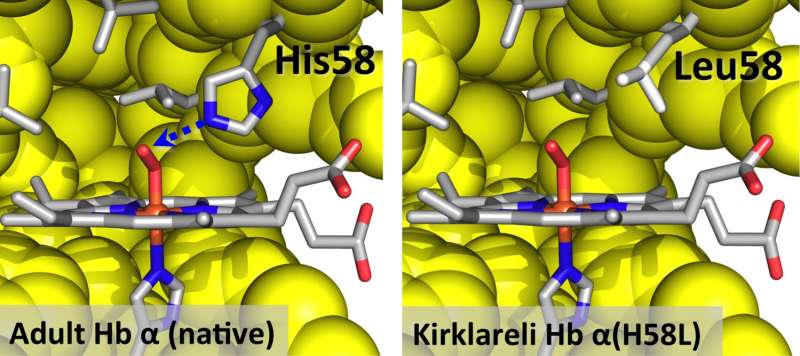
The mutation is in the alpha subunit of human hemoglobin (H58L) and causes it to rapidly auto-oxidize, or rust, which causes the protein to fall apart, lose heme and precipitate. As a result, the protein loses its ability to carry oxygen. Eventually, Olson said, the red cells themselves become deformed and are destroyed.
Remarkably, this same mutation gives the protein an 80,000-fold higher affinity for carbon monoxide than for oxygen. Carbon monoxide from a cigarette will be selectively taken up by the mutant hemoglobin and prevent it from oxidizing and denaturing. This high affinity for carbon monoxide explained why the father showed no signs of anemia, Olson said.
"He may never be an athlete because his blood can't carry as much oxygen, but smoking has prevented him from being anemic," he said. "And there's a side benefit. People with this trait are more resistant to carbon monoxide poisoning."
Olson said he does not know how or if the doctors treated the young woman. He doesn't even know her name. But he suspected her iron-deficient anemia was more an annoyance than a threat to her life and would not recommend she start smoking to relieve it.
"She shouldn't smoke," he said. "But she could take antioxidants, such as a lot of vitamin C, which would help prevent oxidation of her mutant hemoglobin. Her anemia is not that severe. At the same time, she shouldn't worry too much about secondhand smoke, which might have a positive effect."
After ruling out common causes like blood loss, gastritis or congenital defects, her doctors were curious enough about her ailment to call upon Emmanuel Bissé, a researcher at the Institute for Clinical Chemistry and Laboratory Medicine at the University of Freiburg, who discovered the mutation after sequencing her DNA.
Bissé in turn recruited Olson and his team to help determine why the histidine-to-leucine change caused anemia in the daughter but not the father. Ironically, Ivan Birukou, a graduate student in Olson's lab, had already generated the same mutation in human hemoglobin (one of several hundred made at Rice) to study how the protein rapidly and selectively binds oxygen.
"Emmanuel wrote to me and said, 'I know you've been making all these mutants in hemoglobin, and you've probably done the H58L mutation in (alpha) chains. Does this phenotype make sense?'" Olson recalled.
"I said, 'We can do a really neat study here, because we've already made the mutant hemoglobin in a recombinant system.' We actually had a crystal structure (matching Kirklareli) that Ivan and (staff scientist) Jayashree Soman never published but had deposited in the Protein Data Bank. We had made this mutation to try to understand what the distal histidine was doing in alpha subunits."
They found in their 2010 study that replacing the histidine, which forms a strong hydrogen bond to oxygen, with leucine caused a dramatic decrease in oxygen affinity and an increase in carbon monoxide binding. Olson and Birukou realized back then that histidine played a key role in discriminating between oxygen and carbon monoxide in hemoglobin.
"When Emmanuel wrote to me about his discovery, I already 'knew' what was happening with respect to carbon monoxide binding," Olson said.
He said that the normal hydrogen bond causes bound oxygen to stick more tightly to hemoglobin in the same way hydrogen bonds cause spilled soda to feel sticky. "When you touch it, the sugar oxygens and hydrogens make hydrogen bonds with the polysaccharides on your finger," Olson said. "That stickiness helps hold onto oxygen. But leucine is more like an oil, like butane or hexane, and oxygen does not stick well inside hemoglobin. In contrast, bound carbon monoxide is more like methane or ethane and can't form hydrogen bonds."
Andres Benitez Cardenas, a postdoctoral researcher in Olson's laboratory, did the crucial experiment in which he put carbon monoxide on the mutant alpha subunit of hemoglobin Kirklareli. The bound carbon monoxide slowed down oxidation of the protein and prevented loss of heme and precipitation. "In effect, Andres did the 'smoking experiment' to show why the father's hemoglobin didn't denature and cause anemia," Olson said.
He said the effect caused by Kirklareli, though unusual, is not unique.
"There is another 'smoking is good for you' mutation," he said, noting discoveries in Zurich in the late 1970s and early '80s. That case mirrored the current collaboration, as the researchers looking for answers then sought help from Nobel Laureate Max Perutz, whose pioneering work on hemoglobin structures won him the prize in 1968. Olson himself served as a reviewer on some of the papers for hemoglobin Zurich in the 1980s.
"Emmanuel knew that we had worked on these histidine-to-leucine mutations in myoglobin and hemoglobin, which is why he contacted us," he said. "This type of collaboration is how science and medicine should work together."
More information: Emmanuel Bissé et al. Hemoglobin Kirklareli (α H58L), a New Variant Associated with Iron Deficiency and Increased CO Binding, Journal of Biological Chemistry (2017). DOI: 10.1074/jbc.M116.764274
Journal information: Journal of Biological Chemistry
Provided by Rice University