Twisting of protein molecules in water is successfully captured on molecular movie
A research group led by Hyotcherl Ihee at the Korea Advanced Institute of Science and Technology (KAIST) observed twisting of protein molecules in an aqueous solution (which is very similar to the environment in vivo at room temperature) as an X-ray molecular movie with a precision of 100 picoseconds (one over one-ten-billionth of a second). They were assisted by Prof. Shin-ichi Adachi at the Institute of Materials Structure Science at the High Energy Accelerator Research Organization (KEK), Prof. Shin-ya Koshihara at the Graduate School of Science and Engineering at the Tokyo Institute of Technology, and a research group from the University of Chicago in the US.
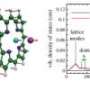
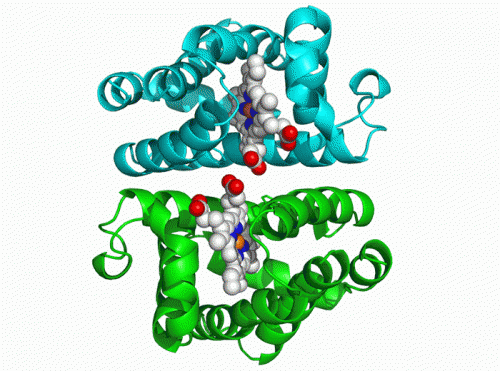
Hemoglobin of clams consists of two weakly bonded units, each of which contains iron porphyrin (heme) complexes. Molecules of oxygen or carbon monoxide reversibly bind to the iron and are thereby carried in the blood. The hemoglobin molecule was irradiated with laser light for a short period of time. The changes in the molecular structure of the protein that occurred after irradiation were tracked at the Photon Factory of KEK, using the time-resolved X-ray solution scattering method. In this study, laser light irradiation was used to break the bonds between the heme complexes in the hemoglobin molecules and carbon monoxide to obtain a transient state in which carbon monoxide is dissociated from the protein. The structure of hemoglobin molecules gradually changed within a timeframe from 100 ps (one-ten-billionth of a second ) to 10 ms (one-hundredth of a second), whereby the distance between the two units became shorter and the two units rotated by approximately 3 degrees relative to each other (Fig. 1).
This is a revolutionary method that can be used to visualize, as a molecular movie, various proteins moving naturally in environments very similar to the environments in vivo. Expectations are high that this new technology will able to analyze the molecular functions of proteins that are important in biological activities.