Oxygen takes elitist attitude to sharing electrons
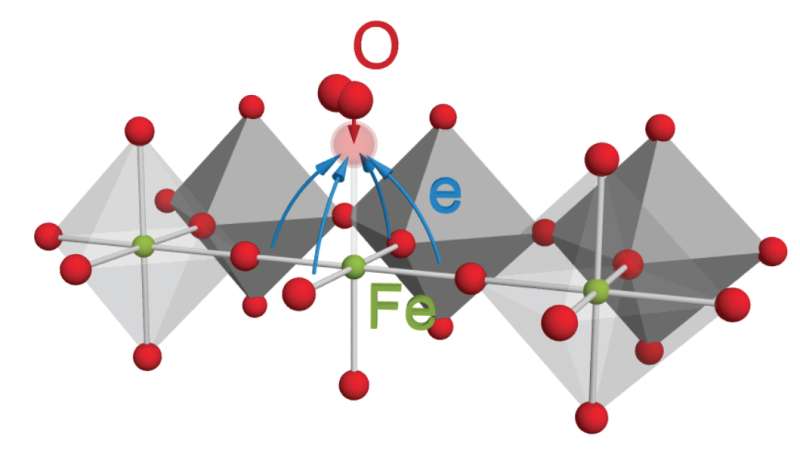
Fuel cells, metal-air batteries, and other devices use electron-exchanging reactions involving gaseous oxygen. To improve these technologies, scientists need to know how the oxygen behaves when it encounters the catalyst, a material that enables the overall reaction. Researchers in California just overturned the conventional thinking about the oxygen's behavior; they showed that the oxygen doesn't exchange electrons with iron and similar metals as once thought. Instead, electron-rich oxygen ions on the catalyst's surface partner with incoming molecular oxygen to break apart the molecule and incorporate the resulting pieces into the catalyst.
Catalysts that coordinate the passage of electrons facilitate reactions in many promising renewable energy technologies, including fuel cells, water splitters, and artificial photosynthesis. By better understanding what happens on the surfaces of these catalysts, scientists can greatly improve these technologies.
The electrochemical reactions of oxygen gas are of great interest in many developing technologies such as solar energy conversion and energy storage. These reactions rely on electrochemical catalysts that are greatly influenced by the surface redox-active centers of the transition-metal oxide material. Measurements of surface redox states would greatly aid the viability of electrocatalysts; however, these states are very sensitive and, thus, difficult to measure with typical surface-sensitive techniques.
To overcome this problem, researchers at Lawrence Berkeley National Laboratory, SLAC, and Stanford University have developed an operando X-ray absorption spectroscopy (XAS) technique that allows scientists to measure redox states while the electrochemical reaction occurs. Using this method, they determined the redox states of various thin-film iron and cobalt perovskite oxides during oxygen gas incorporation and evolution reactions. Results showed that a narrow electronic state of significant oxygen 2p character was responsible for the exchange of electrons with the oxygen molecule adsorbates. This finding implies that oxygen anions near the surface are significant redox partners, which contrasts the conventional idea that the transition metal cations are the dominant redox partners. Not only does this research provide new and valuable information about a specific electrocatalyst material, it also provides a method for further investigation.
More information: David N. Mueller et al. Redox activity of surface oxygen anions in oxygen-deficient perovskite oxides during electrochemical reactions, Nature Communications (2015). DOI: 10.1038/ncomms7097
Journal information: Nature Communications
Provided by US Department of Energy