This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Increasing solid-state electrolyte conductivity and stability using helical structure
Solid-state electrolytes have been explored for decades for use in energy storage systems and in the pursuit of solid-state batteries. These materials are safer alternatives to the traditional liquid electrolyte—a solution that allows ions to move within the cell—used in batteries today. However, new concepts are needed to push the performance of current solid polymer electrolytes to be viable for next generation materials.
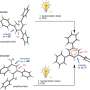
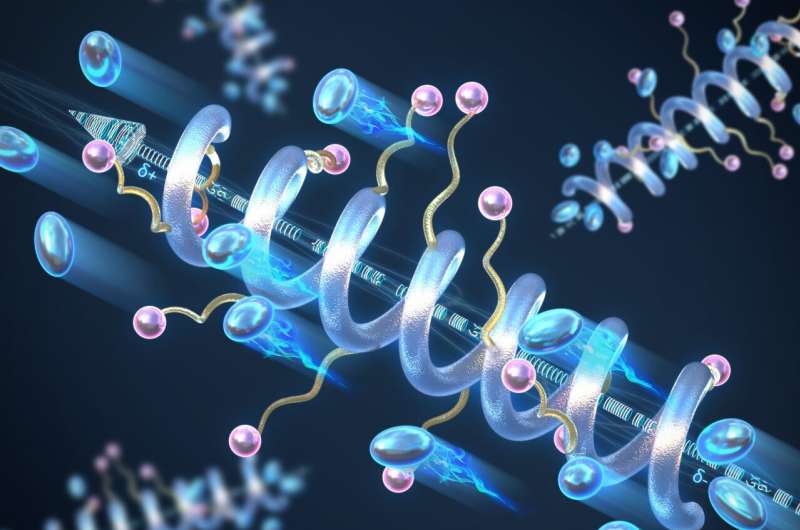
Materials science and engineering researchers at the University of Illinois Urbana-Champaign have explored the role of helical secondary structure on the conductivity of solid-state peptide polymer electrolytes and found that the helical structure shows greatly enhanced conductivity compared to the "random coil" counterparts.
They also found that longer helices lead to higher conductivity and that the helical structure increases the overall stability of the material to temperature and voltage.
Their research, "Helical peptide structure improves conductivity and stability of solid electrolytes," was published in Nature Materials.
"We introduced the concept of using a secondary structure—the helix—to design and improve upon the basic material property of ionic conductivity in solid materials," says Professor Chris Evans, who led this work. "It's the same helix that you would find in peptides in biology, we're just using it for non-biological reasons."
Polymers tend to adopt random configurations, but the backbone of the polymer can be controlled and designed to form a helical structure, like DNA. As a consequence, the polymer will have a macrodipole moment—a large-scale separation of positive and negative charges.
Along the length of the helix, the small dipole moments of each individual peptide unit will add up to form the macrodipole, which increases both the conductivity and dielectric constant—a measure of a material's ability to store electrical energy—of the entire structure and improves charge transport. The longer the peptide, the higher the conductivity of the helix.
Evans adds, "These polymers are much more stable than typical polymers—the helix is a very robust structure. You can go to high temperatures or voltages compared to random coil polymers, and it doesn't degrade or lose the helix. We don't see any evidence that the polymer breaks down before we want it to."
Further, since the material is made from peptides, it can be degraded back into individual monomer units using enzymes or acid when the battery has failed or reached the end of its useful life. The starting materials can be recovered and reused after a separation process, reducing its environmental impact.
Chris Evans is also an affiliate of the Materials Research Laboratory (MRL) and the Beckman Institute for Advanced Science and Technology at Illinois.
More information: Yingying Chen et al, Helical peptide structure improves conductivity and stability of solid electrolytes, Nature Materials (2024). DOI: 10.1038/s41563-024-01966-1
Journal information: Nature Materials