Plastic 'leaves' turn water into fuel
Monash University researchers have developed a new plastic material that can extract hydrogen from water. It could be the start of a water-fuelled energy revolution.
The idea of splitting water (H2O) into hydrogen and oxygen is not a new one. If the two can be split efficiently enough, that hydrogen becomes a valuable potential fuel.
But previous methods to extract the hydrogen from water have either been very expensive or very inefficient, requiring a huge amount of energy to split water and produce hydrogen.
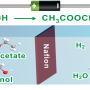
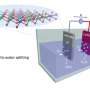
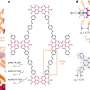
Chun Hin Ng has worked out a way to make this process cheaper, more sustainable and efficient, by using a carbon-based plastic material that can conduct electricity.
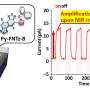
When light is shone onto this plastic, the energy from the light is harnessed to reduce the overall energy requirement to split water into hydrogen and oxygen.
This hydrogen can then react with oxygen to produce 'clean' energy, with the only emissions being water vapour, which can then be put back into the cycle and used again.
It's early days yet – currently, only small amounts of hydrogen have been produced. But this could be the spark of new cheaper, renewable energy.
More information: Chun Hin Ng et al. Exploration and optimisation of poly(2,2′-bithiophene) as a stable photo-electrocatalyst for hydrogen production, J. Mater. Chem. A, 2015,3, 11358-11366, DOI: 10.1039/C5TA00291E , http://pubs.rsc.org/en/content/articlelanding/2015/ta/c5ta00291e#!divAbstract
Journal information: Journal of Materials Chemistry A
Provided by Fresh Science