Towards eco-friendly industrial-scale hydrogen production
What if industrial waste water could become fuel? With affordable, long-lasting catalysts, water could be split to produce hydrogen that could be used to power fuel cells or combustion engines. By conducting complex simulations, scientists showed that adding lithium to aluminum nanoparticles results in orders-of-magnitude faster water-splitting reactions and higher hydrogen production rates compared to pure aluminum nanoparticles. The lithium allowed all the aluminum atoms to react, which increased yields.
The atomistic simulations predicted that hydrogen production could be scaled with the number of surface atoms of nanoparticles. The simulations also provided insights on how the composition of a nanoparticle could improve the efficiency of hydrogen production. These findings advance concepts for nanoparticle-facilitated production of hydrogen at the industrial scale, bringing us closer to an environmentally friendly hydrogen economy.
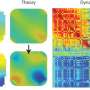
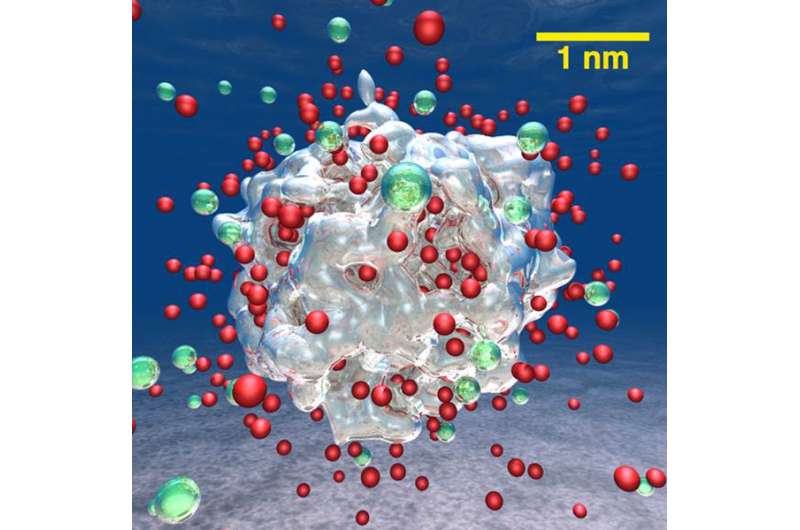
Molecular hydrogen (H2) is an eco-friendly fuel because only waste water is produced when it is burned in combustion engines or used in fuel cells to power cars. The challenge is "Where do we get the hydrogen?" In a sustainable hydrogen production cycle, metallic alloys can split water (H2O) to produce H2. However, ordinary-sized metal particles quickly become blocked from further reactions. Scientists led by the University of Southern California, Los Angeles, have predicted a dramatic increase in hydrogen production from water using alloy nanoparticles based on an equal mixture of aluminum and lithium atoms. During the crucial steps of surface catalysis, metal atoms attract the oxygen (O) atoms in water and release hydrogen. The cycle is completed when the metal is restored to its form without oxygen, which requires energy, e.g., solar or thermal energy. To predict material properties without introducing additional assumptions, large quantum molecular dynamics simulations were performed on 786,432 computer processors for lithium-aluminum nanoparticles. The simulations contained up to 16,000 atoms and explained rapid hydrogen production with higher yields for these nanoparticles in water.
Four specific features were found to reduce the energy required for the reactions already at room temperature: (1) Abundant neighboring pairs of lithium and aluminum atoms are needed to produce hydroxyl (Li-OH) and hydride (Al-H) groups. (2) Lithium atoms transferred charge to the metallic cluster of aluminum atoms forming a negatively charged superanion dispersed with positively charged lithium atoms. (3) Surprisingly, the continuous formation and breaking of bridges by oxygen bonding to lithium and aluminum also played an active role by breaking oxygen-hydrogen bonds and forming aluminum-oxygen bonds. (4) Lithium and OH dissolved and created a more basic solution that inhibited the formation of a reaction-stopping oxide layer on the nanoparticle surface. These atomistic mechanisms not only explain recent experimental findings, but also predict hydrogen production to scale up to industrially relevant particle sizes because of its dependence on the number of surface atoms.
More information: Kohei Shimamura et al. Hydrogen-on-Demand Using Metallic Alloy Nanoparticles in Water, Nano Letters (2014). DOI: 10.1021/nl501612v
Journal information: Nano Letters
Provided by US Department of Energy