How did phosphate get into RNA?
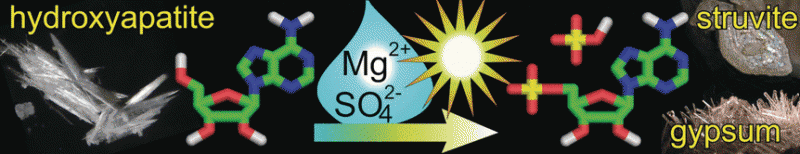
The phosphate ion is almost insoluble and is one of the most inactive of Earth's most abundant phosphate minerals. So how could phosphate have originally been incorporated into ribonucleotides, the building blocks of RNA, which are considered to be among the earliest constituents of life? American and Spanish scientists have now identified reasonable conditions to mobilize phosphate from insoluble apatite minerals for prebiotic organophosphate synthesis, including ribonucleotides. The pivotal role of urea in this process is also described in their article in the journal Angewandte Chemie.
The energy-rich organophosphate bond is one of the basic features found in modern life. Phosphoester and phosphodiester bonds are currently formed using energy from photosynthesis and the energy in our food, and are continuously degraded and reconstructed during metabolic activity within living cells. Phosphate groups also ensure the solubility of RNA and DNA molecules. But how were the very first phosphate ester bonds formed on the prebiotic Earth? Phosphate would have been mostly locked in minerals when the first nucleobases, sugars, and amino acids started life in the primeval soup, which is also known as Darwin's "warm little pond". César Menor-Salván and Nicholas V. Hud at the Georgia Institute of Technology, Atlanta, USA, and collaborators have now explored, in detail, realistic geochemical conditions that could have led to the first relevant organophosphates.
They were especially interested in the role of urea, a hydrolysis product of cyanamide and produced in Miller–Urey type reactions, which also has been shown to catalyze phosphate ester synthesis. The authors hypothesized that a eutectic mixture of urea, ammonium formate, and water could both serve as a milieu for direct phosphorylation and mobilize the phosphate of minerals, and thus allow phosphorylation from mineral sources. Additionally, upon heating formamide is formed, which is a cosolvent and could enhance phosphorylation from mineral sources. Therefore, the eutectic mixture would ensure "a consistent starting concentration of components regardless of their initial abundances," the authors wrote.
Their experiments resulted in effective phosphorylation of nucleosides when heated at moderate temperatures, provided soluble phosphate ions were available. To address the latter point, the scientists added various mixtures of ions and salts to the mixture and observed not only increased solubility of phosphate from hydroxyapatite, but also the formation of moderately soluble secondary phosphate minerals. They wrote: "These experiments suggest that an environment rich in ammonia, small organics such as urea and formate, magnesium sulfate, and phosphate could be an ideal location for prebiotic organophosphate synthesis." Every salt and ion added was very likely abundant in the environment of the prebiotic Earth. Overall, the scientists have identified realistic conditions under which early phosphorylation from insoluble sources could have taken place. So, consistent with Darwin's early thoughts: With the help of urea, phosphorylated molecules important for life can be readily formed in "warm little ponds."
More information: Bradley Burcar et al. Darwin's Warm Little Pond: A One-Pot Reaction for Prebiotic Phosphorylation and the Mobilization of Phosphate from Minerals in a Urea-based Solvent, Angewandte Chemie International Edition (2016). DOI: 10.1002/anie.201606239
Journal information: Angewandte Chemie , Angewandte Chemie International Edition
Provided by Wiley