Cement design that accounts for water confined in the smallest pores
Cement is subjected to a vast range of conditions, both physiological and meteorological, including extreme temperatures, humidity and pressure. Conditions can range from -80 ºC in places such as scientific bases in the Antarctic, to several hundreds of degrees in infrastructure near heat sources or in the case of fires.
These variations in humidity and temperature are translated into physical processes involving evaporation or freezing of the water contained in the cement paste, which often cause stress and even micro-cracking inside the cement. Characterizing the response to these phenomena affecting the confined water in the smallest pores of the cement "is hugely important, as a large proportion of the water, about 30 percent, is located in these small spaces, so to a great extent it contributes towards the final properties of the material," said Hegoi Manzano, a researcher in the UPV/EHU's department of Condensed Matter Physics, and author of the study in collaboration with a research group of the University of Tohoku in Japan.
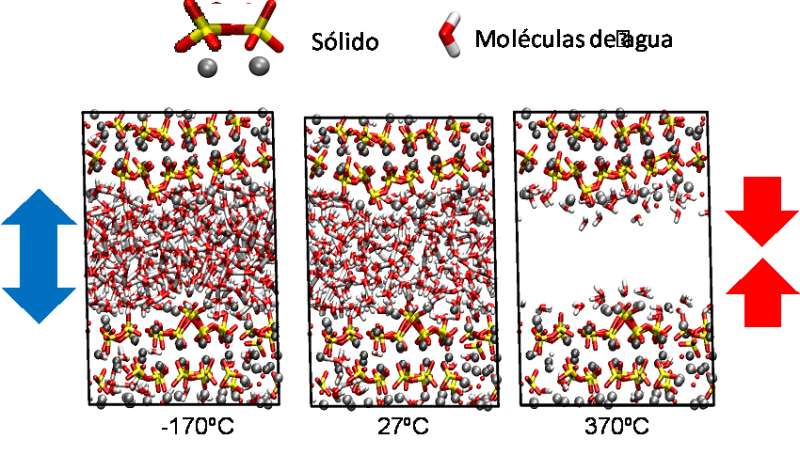
Given the difficulty of studying the behaviour of water located in pores of approximately one nanometre in size by means of experimental channels, the researchers resorted to molecular simulation methods that "imitate" the interactions among the atoms that make up the cement in order to determine how they behave as a whole and the properties that these interactions are translated into. The temperature range the researchers studied was from -170 ºC to 300 ºC.
Stresses at both extremes
The simulations revealed that at both extremes of temperature, "significant volume changes owing to water physics take place. Through totally opposite effects, we arrived at the same consequences," Manzano said. At high temperatures, the water evaporates and disappears from the pores. In these conditions, the pressure brought to bear by the material itself may cause the empty pores to collapse and micro-cracking to occur, which in particularly serious cases, could cause the material to collapse.
At extremely low temperatures, what happens is that the water freezes and therefore expands. "In these conditions, it should be emphasized that the frozen water does not form ice because of the small space in which it is located; the water molecules cannot order themselves to form a crystalline ice structure," Manzano said. However, the expansion is enough to create stresses in the cement and likewise cause micro-cracking.
The conclusions of this study can be used to modify the formulation of the cement for infrastructures that are going to be located in environments with extreme temperatures. "Let us take, for example, an oil company. Knowing the stresses and forces that may be created in the cement, they would have the chance to change certain design factors, such as the additives added to the cement to compensate for the expansion or collapsing of the material in oil wells. That would be the ideal application of the work," concluded Manzano.
More information: Patrick A. Bonnaud et al, Temperature Dependence of Nanoconfined Water Properties: Application to Cementitious Materials, The Journal of Physical Chemistry C (2016). DOI: 10.1021/acs.jpcc.6b00944
Journal information: Journal of Physical Chemistry C
Provided by University of the Basque Country