July 22, 2016 feature
Plant cellulose prevents short circuits in batteries
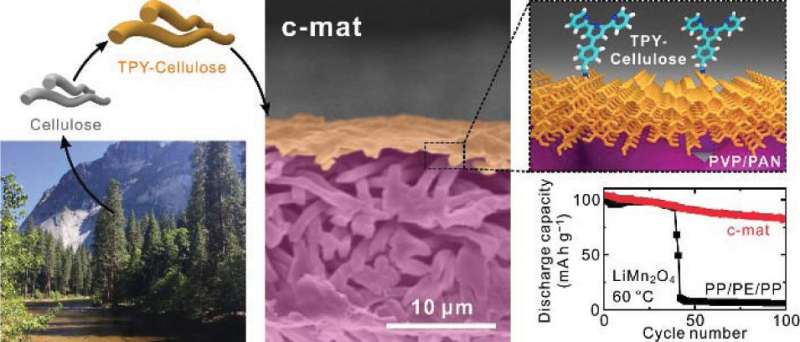
(Phys.org)—In order to prevent short circuits in batteries, porous separator membranes are often placed between a battery's electrodes. There is typically a tradeoff involved, since these separators must simultaneously prevent leakage current between electrodes while allowing ions to pass through the porous channels to generate current. Conventionally, these membranes are made of synthetic materials, such as polymers.
In a new study published in Nano Letters, researchers from the Ulsan National Institute of Science and Technology (UNIST) in South Korea have designed a cellulose nanomat, or "c-mat," separator membrane that contains a thin layer of nanoporous plant cellulose on top of a thick macroporous polymer layer.
By finely tuning the thicknesses of the two layers, the researchers were able to design a separator membrane that delicately balances the tradeoff between preventing leakage current and supporting fast ion transport.
With its tiny pores, the nanoporous cellulose layer prevents leakage current between electrodes, preventing short circuits. On the other hand, the macroporous polymer layer's porous channels are too large to prevent leakage current between electrodes, but their large size enables them to function like "ionic highways" to rapidly transport charges.
The new separator has another major advantage: At high temperatures (60 °C), batteries with the new separator membranes have an 80% capacity retention after 100 cycles, whereas batteries with typical commercial polymer separators maintain just 5% of their initial capacity after 100 cycles at the same temperature.
The researchers explain that the large capacity loss in the commercial batteries at high temperature occurs due to unwanted side reactions between lithium salts and water, which produces harmful byproducts such as manganese ions. The nanoporous cellulose-based layer of the new separator membranes has a manganese-chelating ability, so that it binds to the manganese ions and prevents them from participating in the reactions that cause capacity loss. In addition, the macroporous polymer layer captures the acidic reactants that produce the manganese ions, resulting in fewer of these ions in the first place.
"We demonstrate in this work that the chemically active cellulose-based c-mat separator can mitigate the manganese ion-induced adverse effects," coauthor Sang-Young Lee, Professor at UNIST's School of Energy and Chemical Engineering, told Phys.org. "This enables a remarkable improvement in the high-temperature cycling performance far beyond that which is attainable with conventional membrane technologies."
In the future, the researchers plan to modify the separators for potential use in next-generation rechargeable batteries such as sodium-ion, lithium-sulfur, and metal-ion batteries.
"The c-mat separator is expected to be used for next-generation high-performance batteries with high temperature stability—for example, in large-sized batteries for electric vehicles and grid-scale electricity storage systems," Lee said.
In addition to its use as a battery separator membrane, the c-mat separator also has potential applications in membranes for desalination systems, as well as for ecofriendly sensors for heavy metal ions.
More information: Jung-Hwan Kim et al. "Functionalized Nanocellulose-Integrated Heterolayered Nanomats toward Smart Battery Separators." Nano Letters. DOI: 10.1021/acs.nanolett.6b02069
Journal information: Nano Letters
© 2016 Phys.org



















