New class of protein could treat cancer and other diseases, researchers find
A protein designed by researchers at Georgia State University can effectively target a cell surface receptor linked to a number of diseases, showing potential as a therapeutic treatment for an array of illnesses, including cancer, according to the research team.
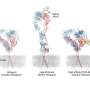
ProAgio, which is created from a human protein, targets the cell surface receptor integrin αVβ₃ at a novel site that has not been targeted by other scientists. The researchers found ProAgio induces apoptosis, or programmed cell death, of cells that express integrin αVβ₃. This integrin has been a focus for drug development because abnormal expression of αVβ₃ is linked to the development and progression of a number of diseases.
The findings are reported in the journal Nature Communications.
"This integrin pair, αVβ₃, is not expressed in high levels in normal tissue," said Zhi-Ren Liu, lead author of the study and professor in the Department of Biology at Georgia State. "In most cases, it's associated with a number of different pathological conditions. Therefore, it constitutes a very good target for multiple disease treatment."
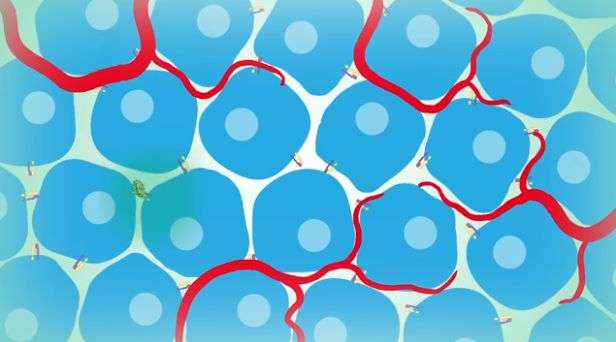
Integrins are cell surface receptors that play a critical role in cells being able to attach to the extracellular matrix. They are composed of different combinations of α and β subunits. Different types of cells have different pairs of subunits.
Integrin αVβ₃ has been studied by many scientists as a potential target for drugs that prevent inflammation and the growth of new blood vessels. This integrin is expressed in the cells of new blood vessels, activated macrophages (immune cells that are involved in the first defense against infection), some cancer cells that metastasize or spread to other parts of the body and bone cells that are critical to maintenance and repair. Previous approaches to targeting this integrin have focused on ligand binding, or attaching a molecule to the active site, which hasn't been effective. There is an urgent need to develop agents that target this integrin at sites other than the ligand-binding site, Liu said.
"We took a unique angle," Lui said. "We designed a protein that binds to a different site. Once the protein binds to the site, it directly triggers cell death. When we're able to kill pathological cells, then we're able to kill the disease."
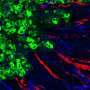
In this study, researchers performed extensive cell and molecular testing that confirmed ProAgio interacts and binds well with integrin αVβ₃. They found ProAgio induces apoptosis by recruiting caspase 8, an enzyme that plays an essential role in programmed cell death, to the cytoplasmic area of integrin αVβ₃. ProAgio was much more effective in inducing cell death than other agents tested.
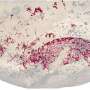
In addition, tests with mouse models of cancer showed ProAgio strongly inhibits tumor growth. Tissue analyses indicated the protein effectively prevents the growth of tumor blood vessels, while existing blood vessels were not affected. Toxicity tests also showed that ProAgio is not toxic to normal tissue and organs in mice.
Journal information: Nature Communications
Provided by Georgia State University