November 20, 2015 report
New study shows inhibiting copper chaperones reduces tumor cell proliferation
(Phys.org)—Researchers from several institutions have found that copper trafficking chaperones are a good target for suppressing tumor growth without affecting healthy cells.
By inhibiting key copper chaperones, Atox1 and CCS, copper accumulated in cancer cells, leading to oxidative stress and lipid biosynthesis inhibition which, in turn, inhibited cancer cell proliferation. Importantly, small molecule inhibition of Atox1 and CCS affected cancer cells without showing detrimental effects in healthy cells as determined in both in vitro human cell and in vivo mouse studies. This work is reported in Nature Chemistry.
Cancer cells are known to have more copper than normal cells. Intercellular copper transport is a highly regulated cellular process. Too much copper can be toxic for cells, but copper is necessary for cellular function. Why cancer cells accumulate copper and what this does to the cells is largely unknown.
Prior research with copper chelators showed some positive effects in inhibiting tumor growth, but chelators are non-specific and bind copper both within and outside of the cells, leading to toxic effects.
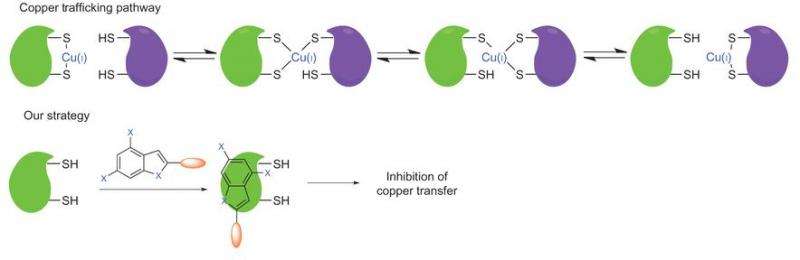
Another place of attack in the copper transport pathway is the proteins involved in copper transport. Atox1 and CCS are two copper chaperones whose knockdown led to reduced tumor proliferation due to inhibition of cellular copper trafficking.
To that end, Wang, et al. found a small molecule that inhibits both Atox1 and CCS. Because inhibiting these proteins does not change the extracellular copper concentration, they were able to investigate the mechanism behind tumor inhibition by blocking copper delivery inside cells.
To find their small molecule, they conducted a virtual screening of molecules with the appropriate characteristics to preferentially bind to the Atox1 and CCS copper-binding sites. After validating their results with FRET studies and testing with lung cancer (H1299), head and neck cancer (212LN), and breast cancer (MB231) cell lines, they found two molecules (DC_AC2 and DC_AC50) that preferentially kill the cancerous cells without killing normal cells. In vivo studies with mice showed that DC_AC2 was toxic, so the focus of their studies was on DC_AC50. DC_AC2 is very similar in structure and binding to DC_AC50 and is soluble in water, so it was used in some studies to elucidate cellular mechanisms.
Because DC_AC50 is intrinsically fluorescent, binding affinities could be done suing FRET and fluorescence anisotrophy studies. Additionally NMR titrations were used to verify the fluorescence studies. The results of these studies confirmed that copper is not required for DC_AC50 to bind to Atox1 and CCS, and mutation studies verified that DC_AC50 directly binds to the copper transfer interfaces of the chaperones.
Cellular studies verified that the presence of DC_AC50 reduces cancer cell proliferation. Control studies using an inactive analog to DC_AC50 as well as control studies with normal epithelial lung cells and breast cells revealed that DC_AC50 affects cancer cells, while only having a minimal effect on normal cells. Furthermore, these studies confirmed that inhibiting both Atox1 and CCS inhibits cells growth, verifying that both are involved in copper transport in cancer cells.
Injecting varying dosages of DC_AC50 showed a decrease in tumor cell growth, confirming that is dose-dependent and minimally toxic at doses of 10-50 mg/kg per day in animal studies.
The cellular mechanisms at play here are both an increase in oxidative stress (increasing ROS levels) and hindering lipid synthesis. Copper accumulation affects copper trafficking across the mitochondrial membrane by interfering with the oxidative phosphorylation pathway. This results in decreased ATP production as well as increasing ROS levels via reduced COX activity due to Atox1 and CCS inhibition. This process leads to AMPK activation, which signals the phosphorylation of acetyl-CoA-carboxylase 1. Phosphorylation of AMPK and ACC1 are important regulators that halt lipid biosynthesis. This cascade effect results in tumor growth inhibition.
By blocking two chaperone proteins in copper transport, Wang, et al. demonstrated that the cellular effects of copper accumulation serve to inhibit tumor growth while only having a minimal effect on normal cells. Additionally, they were able to show which cellular mechanisms were involved.
More information: Jing Wang et al. Inhibition of human copper trafficking by a small molecule significantly attenuates cancer cell proliferation, Nature Chemistry (2015). DOI: 10.1038/nchem.2381
Abstract
Copper is a transition metal that plays critical roles in many life processes. Controlling the cellular concentration and trafficking of copper offers a route to disrupt these processes. Here we report small molecules that inhibit the human copper-trafficking proteins Atox1 and CCS, and so provide a selective approach to disrupt cellular copper transport. The knockdown of Atox1 and CCS or their inhibition leads to a significantly reduced proliferation of cancer cells, but not of normal cells, as well as to attenuated tumour growth in mouse models. We show that blocking copper trafficking induces cellular oxidative stress and reduces levels of cellular ATP. The reduced level of ATP results in activation of the AMP-activated protein kinase that leads to reduced lipogenesis. Both effects contribute to the inhibition of cancer cell proliferation. Our results establish copper chaperones as new targets for future developments in anticancer therapies.
Journal information: Nature Chemistry
© 2015 Phys.org