Researchers develop pioneering new method to map enzyme activity
Researchers from Cardiff University have pioneered a new technique that will enable scientists to precisely pinpoint the areas on an enzyme that help to speed up chemical reactions.
By labelling certain segments of an enzyme with heavy isotopes, the researchers have found that 'heavy' and 'light' versions of enzymes have different catalytic properties, allowing them to determine which regions are linked to specific functions.
It is hoped this precise pinpointing may shed light on why enzymes are much more efficient at speeding up chemical reactions compared to man-made catalysts, and could have wide-reaching implications for a range of industries, such as the creation of manufactured goods, biofuels and therapeutic drugs.
Lead author of the study Professor Rudolf Allemann, distinguished research professor and head of Cardiff University's School of Chemistry, said: 'Enzymes are not only central to living systems, but also to many industrial processes, such as the production of food, textiles, detergents, pharmaceuticals and other chemicals where environmentally friendly methods are of ever increasing importance.
'We've been able to show, for the first time, that different parts of an enzyme affect different aspects of its function. The method we've developed can be applied to a wide range of pharmaceutically and industrially important enzymes, and will lead to new candidates for biological and medical applications, and new production routes for enzymes of industrial use.'
Enzymes are proteins that drive nearly all chemical reactions that take place in living systems.
Researchers around the world are striving to understand exactly how enzymes increase reaction rates, as they typically operate much more efficiently, and in more friendly conditions, than man-made catalysts that are used in industry. It is estimated that man-made catalysts underpin the creation of 80 to 90 percent of all manufactured goods.
Whilst researchers have a good understanding of the chemistry of enzymes, they are less sure about how enzymes physically react with their targets, specifically how the movements or vibrations of an enzyme can drive the chemical reactions.
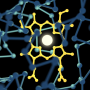
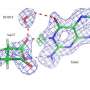
In their study, the research team, consisting of researchers at Cardiff University's School of Chemistry, the University of Valencia and Jaume I University in Spain, investigated the physical movements of the enzyme dihydrofolate reductase (DHFR).
DHFR is a small enzyme that plays an essential role in the building of genetic material and proteins, and was the first enzyme to be targeted for chemotherapy cancer treatment. Drugs were designed to bind strongly to DHFR to prevent it from working, which would stop rapidly reproducing cells—such as cancer cells—from proliferating.
The researchers altered the weight of DHFR by adding heavy isotopes—specifically carbon, nitrogen and hydrogen—onto certain segments of the enzyme. As a result of the additional weight the enzyme moved slower, but its chemical properties remained unchanged.
By strategically altering different parts of the enzyme and recording how the chemical reactions changed, the researchers were able to determine the dynamic role that specific regions of the enzyme played in chemical reactions.
The study has been published in the journal Angewandte Chemie.
Journal information: Angewandte Chemie
Provided by Cardiff University