Core work: Iron vapor gives clues to formation of Earth and Moon
Recreating the violent conditions of Earth's formation, scientists are learning more about how iron vaporizes and how this iron rain affected the formation of the Earth and Moon. The study is published March 2 in Nature Geoscience.
"We care about when iron vaporizes because it is critical to learning how Earth's core grew," said co-author Sarah Stewart, UC Davis professor of Earth and Planetary Sciences.
Shock and release
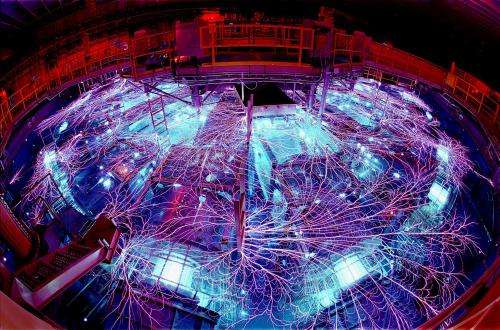
Scientists from Lawrence Livermore National Laboratory, Sandia National Laboratory, Harvard University and UC Davis used one of the world's most powerful radiation sources, the Sandia National Laboratories Z-machine, to recreate conditions that led to Earth's formation. They subjected iron samples to high shock pressures in the machine, slamming aluminum plates into iron samples at extremely high speeds. They developed a new shock-wave technique to determine the critical impact conditions needed to vaporize the iron.
The researchers found that the shock pressure required to vaporize iron is much lower than expected, which means more iron was vaporized during Earth's formation than previously thought.
Iron rain
Lead author Richard Kraus, formerly a graduate student under Stewart at Harvard, is now a research scientist at Lawrence Livermore National Laboratory. He said the results may shift how planetary scientists think about the processes and timing of Earth's core formation.
"Rather than the iron in the colliding objects sinking down directly to the Earth's growing core, the iron is vaporized and spread over the surface within a vapor plume," said Kraus. "This means that the iron can mix much more easily with Earth's mantle."

After cooling, the vapor would have condensed into an iron rain that mixed into the Earth's still-molten mantle.
To the moon
This process may also explain why the Moon, which is thought to have formed by this time, lacks iron-rich material despite being exposed to similarly violent collisions. The authors suggest the Moon's reduced gravity could have prevented it from retaining most of the vaporized iron.
More information: Nature Geoscience, 10.1038/ngeo2369
Journal information: Nature Geoscience
Provided by UC Davis