Scientists find unsuspected spectroscopy rules for rattling hydrogen
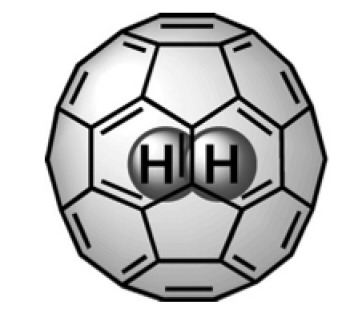
It has been taken for granted for over 50 years that the type of spectroscopy widely used to study hydrogen inside materials is not subject to any selection rules. In the joint theoretical and experimental study that appeared recently in the journal Physical Review Letters, an international team of researchers led by Zlatko Bačić, a professor of chemistry at NYU, showed that this near universally held view is incorrect for at least one important class of hydrogen-entrapping compounds, by confirming experimentally the validity of the selection rule formulated in 2013 by the Bačić group.
Hydrogen holds a great promise as the "green" fuel of the future, since it burns clean, producing only water. But fulfilling this tantalizing potential hinges on finding ways to store hydrogen efficiently, safely, and cheaply. One option that has been investigated intensely by scientists world wide is to trap hydrogen molecules inside nanosized (one billionth of a meter) cavities of various porous materials.
The behavior of light hydrogen molecules squeezed in such tiny spaces is no longer described by classical mechanics, which governs the motions of objects that we encounter in everyday life. Instead, it is governed by quantum mechanics, which rules the domain of atoms and molecules. Accurate understanding of the highly non-classical dynamics of hydrogen entrapped in nanoscale cavities, and how it interacts with host materials, is essential for designing more efficient and economical hydrogen storage media.
A general and very powerful tool for achieving this is spectroscopy. In the case of hydrogen, the most versatile and highly selective is the so-called inelastic neutron scattering (INS) spectroscopy. In the INS, neutrons with precisely known energies are scattered from the entrapped guest hydrogen molecules, causing transitions between the energy levels associated with the "rattling'' of the caged hydrogen.
This gives rise to the INS spectrum, a collection of peaks that in principle contains a wealth of information about the quantum dynamics of hydrogen and the guest-host interactions. However, extracting this valuable information from the spectra is not easy. The key concept in spectroscopy in general is that of selection rules, which determine whether transitions between certain pairs of states of a system are forbidden, and therefore should be absent from the spectra, or allowed. Knowing the selection rules is crucial for the correct interpretation of the spectra; incorrect assumptions about them will inevitably lead to wrong conclusions.
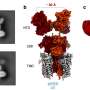
For decades, it has been an article of faith that INS spectroscopy of molecular compounds is free from any selection rules, and this has been cited as one of its strengths and advantages. Then in 2013, the group of Bačić derived theoretically a selection rule for the INS spectroscopy of H2 inside the almost spherical cage of the "buckyball'' molecule C60 (H2@C60), the first ever to be predicted in the INS of any kind of molecular system. H2@C60 has fascinated researchers as a felicitous union of the simplest of all molecules, H2, with one of the most elegant, highly symmetric, and intellectually captivating molecules, C60. According to the newly derived selection rule, certain well-defined transitions in the INS spectra of H2@C60 are forbidden and as such should not be present in the spectra, contradicting the common wisdom that all INS transitions are allowed. Establishing for the very first time the existence of an INS selection rule made it essential to provide its experimental confirmation.
For this purpose, an international team was assembled which, in addition to the theory group of Bačić, included some of the world's leading experimental INS groups at the University of Nottingham in UK, Institut Laue-Langevin in Grenoble, France, and the University of Southampton, UK. The INS spectra of H2@C60 were recorded under high resolution in Grenoble, on one of the best neutron scattering spectrometers in existence. These high-quality spectra revealed that all INS transitions accessible experimentally, which our selection rule predicts to be forbidden, are indeed systematically absent from the measured spectra. This provided definitive and complete experimental validation of the theoretically predicted selection rule.
In addition to setting an important precedent, the new selection rule sends a signal to the broad INS community that hitherto unsuspected selection rules may exist in the INS of many important types of discrete molecular compounds, requiring a profound change, and great care, in how such spectra are analyzed and interpreted in the future.
Journal information: Physical Review Letters
Provided by New York University