Wild molecular interactions in a new hydrogen mixture
Hydrogen—the most abundant element in the cosmos—responds to extremes of pressure and temperature differently. Under ambient conditions hydrogen is a gaseous two-atom molecule. As confinement pressure increases, the molecules adopt different states of matter—like when water ice melts to liquid and then heats to steam. Thus far, at extreme pressures hydrogen has four known solid phases. Now scientists, including Carnegie's Alexander Goncharov, have combined hydrogen with its heavier sibling deuterium—which has an added neutron in its nucleus—and created a novel, disordered, "Phase IV"-material where the molecules interact differently than have been observed before.
The new results, published in the October 21, issue of Physical Review Letters, could be valuable for controlling superconducting and thermoelectric properties of novel hydrogen- bearing materials.
Phase IV of dense, solid, pure hydrogen (H2) and deuterium (D2) was previously discovered by several members of the same team and others. The hydrogen molecules exhibited two very different behaviors. One weakly interacted with its neighboring molecules, while the other strongly bonded with its neighbors, forming hexagonal atomic sheets like graphene, a novel truly two-dimensional form of carbon with fascinating electronic properties. Electronically, these layers behave somewhat like a semiconductor and a semimetal. Semimetals are in between metals and semiconductors with respect to their electronic properties.
This team, led by Ross Howie of the University of Edinburgh, combined experiments and theoretical calculations. They mixed the H2 and D2 in varying concentrations and subjected them to room temperature under different pressures, ranging from about 2,000 times atmospheric pressure (.2 GPa) to about 2.7 million atmospheres (270 GPa).
Goncharov explained: "Before conducting the experiments, we thought that the material could change under pressure by several different processes. The mass differences of the molecules mean that they have very different low energy states, which would affect the outcome. In one scenario, the physics could result in the ordered segregation of the H2 and D2 molecules between strongly and weakly bounded layers."
Under another scenario, the molecules might be randomly, or disorderly, distributed. Then there is another intriguing prospect they entertained—whether the disordered state affects the waves of atomic vibrations (called phonons) and prevents them from freely propagating, a phenomenon called Anderson localization. Typically, electrons in solids have energy values only within certain ranges. The scientists thought that vibrational wave propagation through a molecular maze might break this energy band depending on the strength of the molecular bonds, the masses, or both, and could affect just a few, local molecules.
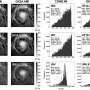
The scientists used a technique called Raman spectroscopy, which measures the tiny quantum behavior of vibrational energy, rotational energy, and other motion in a molecular system when a laser light interacts with the molecules. They then confirmed their experiments with theoretical calculations.
The scientists found that above 1.9 million atmospheres, the vibrational waves show Anderson localization. The extent of this localization depends on the concentration of H2 and D2 and whether these molecules belong to weakly or strongly bound layers. For instance in one layer, H2 molecules vibrated in separate groups of 2 to 3 molecules at frequencies that weakly depended on the neighboring environment. As the hydrogen concentration increased, the different H2 clusters grew and started to couple. This is the first study where Anderson localization from vibrational energy has been observed by interacting with mass differences in a material.
Goncharov remarked, "The Anderson localization of vibrational excitations in hydrogen mixtures provides a new mechanism for optimizing thermoelectric and electronic behaviors, for example in superconductivity."
Journal information: Physical Review Letters
Provided by Carnegie Institution for Science