Bacterial enzyme controls the degradation of defective proteins
A study by RIKEN researchers has revealed an important coping mechanism employed by enteric bacteria that allows them to thrive in environments with and without oxygen. The findings could help develop ways to control both harmful and beneficial bacteria in the human gut.
Most organisms are either oxygen-breathing 'aerobes' or oxygen-avoiding 'anaerobes'. The enteric bacteria that dwell within our gut, however, have specialized biological mechanisms that allow them to live comfortably in environments with and without oxygen.
The research team, led by Wataru Nishii and Shigeyuki Yokoyama from the RIKEN Structural Biology Laboratory, focused on a protease enzyme called Lon, which maintains cellular health by destroying damaged or defective proteins. Virtually all organisms have a version of Lon, but the enzyme found in enteric bacteria differs slightly from those found in other organisms, possessing a pair of amino acids that can form a strong chemical bond under certain chemical conditions.
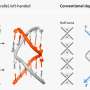
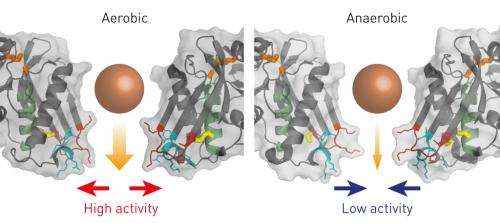
Nishii and Yokoyama investigated the function of this bond by resolving the atomic-scale structure of Lon in its two states: with the two amino acids joined or separated. Protein targets enter Lon through a pore in one surface of the enzyme (Fig. 1), and the digested remnants are then released through a second exit pore. The researchers discovered that in Lon's 'oxidized' state, where the chemical bond is in place, the exit pore is considerably wider than it is in the 'reduced' state, in which the bond is broken.
This subtle shift has important consequences. The narrower pore of the reduced form limits the release of protein fragments, and thereby stalls enzymatic processing. Importantly, the protein can freely shift back and forth between the oxidized and reduced states. Within the oxygen-free anaerobic environment of the gut, the reduced state is favored, resulting in lower Lon activity. Outside the body of a host, the presence of oxygen promotes the oxidation of Lon and greatly boosts enzymatic activity—a protective measure that limits accumulation of damaged proteins during aerobic respiration. "This tiny and simple structural change of Lon directly links molecular functions with environmental conditions," explains Nishii, "thereby ensuring the robust survival of enteric bacteria."
The team intends to explore the regulation of Lon activity further and perhaps identify compounds that might sabotage the adaptability of harmful enteric bacteria such as Escherichia coli and Salmonella. "We want to understand the full details of the linkage between bacterial protein degradation and environmental conditions," says Yokoyama.
More information: Nishii, W., Kukimoto-Niino, M., Terada, T., Shirouzu, M., Muramatsu, T., Kojima, M., Kihara, H. & Yokoyama, S. "A redox switch shapes the Lon protease exit pore to facultatively regulate proteolysis." Nature Chemical Biology 11, 46–51 (2015). DOI: 10.1038/nchembio.1688
Journal information: Nature Chemical Biology
Provided by RIKEN