Metal model mimics metalloenzymes
(Phys.org) —Metal ions play critical roles throughout biochemistry, often facilitating the cleavage of the bond between the two atoms in an oxygen molecule in metalloenzymes. They are the key to oxidizing organic molecules and, in the case of photosynthesis, water. An international team of scientists carrying out research at the U.S. Department of Energy Office of Science's Advanced Photon Source (APS) has homed in on the role of metal ions in a wide range of biological processes, from metabolism to photosynthesis. Their published results were the cover article for the May 1, 2013, issue of the Journal of the American Chemical Society.
The metal ions in question are not themselves redox active, meaning they can grab a single oxygen atom without suffering interfering side reactions. Commonly, these species are hooked up to a heme-type porphyrin molecule, the central unit in the blood protein hemoglobin and the light-sensitive unit in chlorophyll at the heart of photosynthesis.
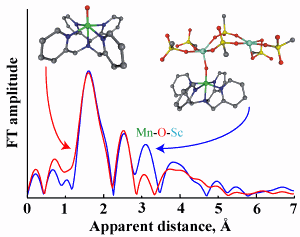
The researchers from Ewha Womans University (Korea), Purdue University, Osaka University (Japan), and the Japan Science and Technology Agency built a simplified model of the natural metal-oxo compound (an oxygen atom bound only to one or more metal centers) using manganese in the IV oxidation state to which is attached a chemical group, or ligand, containing five nitrogen atoms—N,N-bis(2-pyridylmethyl)-N-bis(2-pyridyl)methylamine—which allow it to bond to the manganese(IV) through five points, with the final bonding point available for the oxo, or oxygen atom. Linking scandium ions to this unit completes the structure.
The team used various analytic methods, including electrospray ionization mass spectrometry (ESI-MS), electron paramagnetic resonance spectroscopy, and x-ray crystallography at X-ray Science Division x-ray beamline 20-BM at the Argonne National Laboratory APS to investigate the structure of their model in detail. They also experimented with its chemical reactivity. The binding of the scandium ions accelerates oxidation reactions by as much as 2200 times in the oxidation reaction of thioanisole, an oxygen atom transfer akin to those seen in many enzyme reactions in which the bond between two oxygen atoms is cleaved. The same metal model slows the rate of hydrogen atom transfer in activation of the organic compound 1,4-cyclohexadiene; this also emulates the behavior of the metal-oxo units in the metalloenzymes that the team hopes to understand.
The researchers explain that the precise behavior of their metal-oxo model is tuned by the presence or otherwise of the scandium ions. This simulates the tuning behavior of metalloenzymes by calcium ions in biology, for instance. The presence of calcium ions is essential in the photosynthetic oxidation of water, but their exact role is not entirely clear. The team's demonstration of this tuning process by scandium ions hints at one possible explanation.
A mechanism for why the oxygen atom transfer reaction is accelerated so substantially hints at the involvement of a fast-moving electron transfer pathway, the team explains. Conversely, the slower rate of the hydrogen atom transfer they interpret as being due to the bulk of the scandium(III) ions hindering movement of the hydrogen atoms, which are themselves much, much slower moving than electrons.
Bioinorganic chemist Jon McMaster of the University of Nottingham (UK), an expert in the role of metals in biology, believes the work has clear impact. "This paper is important because it demonstrates that Mn(IV)-O reactivity in oxidation reactions can be altered by binding Sc(III) ions," he says. "This provides insight into the role of redox inactive metal ions in modulating the activity of Mn-oxo species, which may inform the ongoing discussion about the role of the Ca(II) ion in the Mn4CaO5 oxygen evolving center in photosystem II (PSII)," McMaster says.
More information: Chen, J. et al. A Mononuclear Non-Heme Manganese(IV)–Oxo Complex Binding Redox-Inactive Metal Ions, J. Am. Chem. Soc. 135, 6388-6391 (2013). DOI: 10.1021/ja312113p
Journal information: Journal of the American Chemical Society
Provided by Argonne National Laboratory