Scientists determine activation barrier in ammonia-sulfuric acid clusters that could lead to cloud formation
(Phys.org) —Ammonia must overcome an energy barrier to join sulfuric acid and water to create clusters that can lead to cloud formation, according to scientists at Pacific Northwest National Laboratory and the University of Delaware.
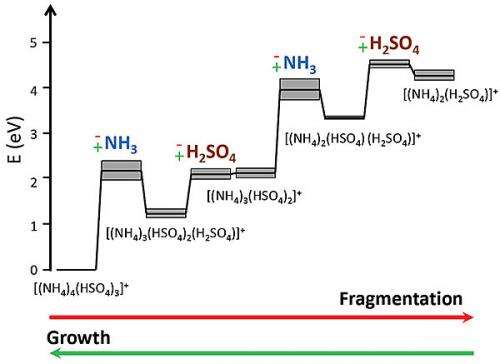
The team used surface-induced dissociation, which breaks apart molecules under controlled conditions. They found that when the clusters fragmented, they either lost an ammonia molecule followed by a sulfuric acid molecule, or lost the two molecules simultaneously. The energy required to dissociate a cluster is higher than the energy of the final products. This energy requirement implies that there is an energy or activation barrier that must be overcome for an ammonia molecule to join the cluster and help it grow. The research also suggests that the more conventional and simple-to-calculate diffusion rate should not be assumed to be the growth rate.
Acidic and basic gases released by gasoline-powered engines, coal-fired power plants, and other sources form clusters of sulfuric acid, water, and ammonia. These clusters can come together to create and grow clusters, which eventually grow large enough to be particles that can serve as the seeds for clouds. Because clouds influence the climate, scientists are developing the most accurate models possible to analyze the impact of regulations and different scenarios. This work paves the way for climate and cloud models with improved accuracy.
The team evaluated the dissociation of two positively charged ammonium bisulfate clusters, which are similar to clusters found in the atmosphere. Surface-induced dissociation of these ammonium bisulfate clusters indicated two unique pathways for cluster fragmentation:
1. a two-step pathway, where a cluster initially loses an ammonia molecule then a sulfuric acid molecule
2. a one-step pathway that proceeds via the loss of an ammonium bisulfate molecule.
The team compared experimental data to quantum chemical calculations of cluster dissociation thermodynamics and determined that the loss of either an ammonia molecule or an ammonium bisulfate molecule is greater than the corresponding thermodynamic value. These results suggest the presence of an activation barrier for ammonia incorporation into molecular clusters as they grow, which may impact cluster distributions in the atmosphere.
The team is continuing to address the formation and growth of the sulfuric acid-ammonia clusters, including examining the height of the activation barrier, the effect of polarity, and the role of water. Further, the team is investigating whether an activation barrier can exist in larger clusters, and if so, how does it affect the cluster's reactivity.
More information: Bzdek, B. et al. 2013. Fragmentation Energetics of Clusters Relevant to Atmospheric New Particle Formation, Journal of the American Chemical Society 135(8):3276-3285. DOI: 10.1021/ja3124509
Journal information: Journal of the American Chemical Society
Provided by Pacific Northwest National Laboratory