Theory models, EMSL capabilities illuminate how particles grow in the atmosphere
Determining the chemical mechanisms that govern new particle formation, or NPF, in the atmosphere is not something that can be pulled out of thin air. In the atmosphere, nucleating clusters are presumed to be composed of a few common species: sulfuric acid (the key chemical component), ammonia, amines (ammonia derivatives), and water—all of which have different effects on nucleation and particle growth. Moreover, these same clusters may significantly impact cloud condensation nuclei, the particles that spawn cloud formation, and, in turn, affect global climate. Scientists used EMSL's Fourier transform ion cyclotron resonance mass spectrometer equipped with surface-induced dissociation (SID) to examine and model fragmentation kinetics and energetics of small clusters that may serve as precursors to NPF.
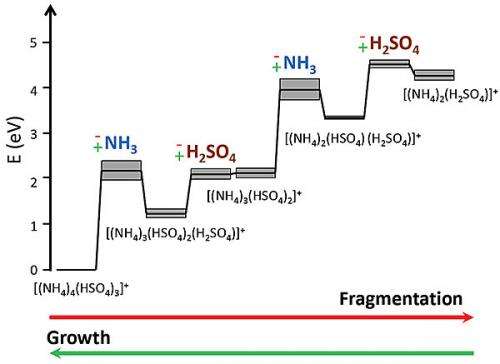
Primarily, they evaluated the role of two positively charged ammonium bisulfate clusters (other studies have shown sulfuric acid−ammonia cluster growth follows an ammonium bisulfate pathway). SID of these ammonium bisulfate clusters indicated two unique pathways for cluster fragmentation: 1) a two-step pathway, where a cluster initially loses an ammonia molecule then a sulfuric acid molecule, and 2) a one-step pathway that proceeds via the loss of an ammonium bisulfate molecule. They compared experimental data to quantum chemical calculations of cluster dissociation thermodynamics and determined that loss of either an ammonia molecule or an ammonium bisulfate molecule is greater than the corresponding thermodynamic value.
These results suggest the presence of an activation barrier for ammonia incorporation into molecular clusters as they grow, which may impact cluster distributions in the atmosphere. Current atmospheric particle models typically do not consider activation barriers in NPF growth processes. Thus, this work paves the way for atmospheric NPF models with considerably improved accuracy.
More information: Bzdek, B. et al. 2013. Fragmentation Energetics of Clusters Relevant to Atmospheric New Particle Formation. Journal of the American Chemical Society 135(8):3276-3285. DOI: 10.1021/ja3124509.
Journal information: Journal of the American Chemical Society
Provided by Environmental Molecular Sciences Laboratory