Cell movement explained by molecular recycling
Scientists at The University of Manchester have identified the method by which cells control the recycling of molecules, a process that is essential for them to move. The discovery provides researchers with a better understanding of how our bodies heal wounds.
Working under Professor Martin Humphries, the Dean of the Faculty of Life Sciences, Dr Mark Morgan and his team at the Wellcome Trust Centre for Cell Matrix Research studied the role of integrins. These molecules are able to grab hold of the fibres surrounding the cell, like hands, allowing the cell to drag its self along. However, there are several types of integrin on the cell surface and they all have different properties which affect how quickly the cell can move.
Once they have been used by the cell, integrins are moved from the surface to a store inside the cell. When the time is right they are recycled back to the cell surface where they can bind with the surrounding fibres once again.
What the team uncovered is the method by which cells dynamically control the type of integrins that are recycled. They found that another molecule on the surface of the cell, called syndecan-4, is able to detect and interpret subtle changes in the cell's surroundings to decide how it should respond. By regulating where and when the different integrins are delivered to the cell surface, syndecan-4 precisely regulates cell movement and exploration.
Dr Morgan says: "Syndecan-4 plays a critical role in regulating wound healing, so ultimately, we hope that this work will inform the development of novel therapeutic strategies to improve wound healing."
Most cells in the body are able to crawl through the dense network of fibres that surround them. This migration process is essential for repairing wounds, tracking down infection and maintaining tissue function.
In order for a cell to move efficiently, it needs to precisely control which integrins are able to bind to the fibres. At certain times and places they need to bind strongly, whereas at other points they need to bind more weakly, and only when these processes are regulated appropriately can a cell migrate properly.
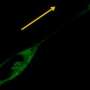
By studying the movement of fibroblast cells using sophisticated imaging techniques, Dr Morgan and the team identified the role of Syndecan-4. They found that it decodes the vast array of signals outside the cell and functions as a molecular switch to dictate whether the strong or weak binding integrins are recycled.
Dr Morgan explains: "When we changed the way Syndecan-4 senses the environment outside the cell, we were able to alter the way that it transmits signals into the cell and control integrin recycling. By manipulating the molecules in this way we found that we could either force the cells to move in a fast forward motion or stop altogether."
Their findings have been published in the journal Developmental Cell.
The next step will be to investigate how Syndecan-4 can be manipulated to control cell movement with a view to developing novel therapeutic strategies to improve wound healing. It will also be important to test whether this mechanism is involved in tumour progression and metastasis as disruptions in cell movement are often seen in cancer, as well as in vascular disorders and chronic inflammatory disease.
More information: The paper has been published in the journal Developmental Cell and is titled "Syndecan-4 phosphorylation is a control point for integrin recycling".
Journal information: Developmental Cell
Provided by University of Manchester