Researchers identify structure of circadian clock protein
(PhysOrg.com) -- Feeling jet-lagged? You may need your internal clock reset. New Cornell research has taken a major step toward treating jet lag and other more serious syndromes by advancing our understanding of how circadian rhythms work.
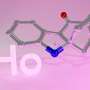
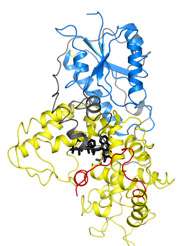
Cornell researchers have identified for the first time the 3-D crystal structure of a protein in fruit flies (Drosophila) that also facilitates circadian rhythm functions in most higher organisms -- from cyanobacteria and plants to animals, including humans.
The study appears in the Nov. 13 issue of the journal Nature.
While the mechanisms of circadian -- or biological -- clocks are complex in humans, many of their key components are shared by such lower species as Drosophila, which serve as a model organism for understanding circadian rhythms.
The protein, called cryptochrome (dCRY), plays a key role in circadian clocks, which get cues from daylight and allow organisms to pace their metabolism on a 24-hour cycle.
Biological clocks regulate such processes as opening and closing of petals and shedding of leaves in plants, for example, and the timing of hunger, waking, excretion and changes in blood pressure in people. Minor disruptions of human circadian clocks can lead to jet lag and fatigue, while chronic malfunctions have been associated with some mental illnesses and cancers.
"The aim of the study was to understand the structure of dCRY at the molecular level," which was done by using X-ray crystallography, said co-author Anand Vaidya, a graduate student working in the lab of senior author Brian Crane, professor of chemistry and chemical biology. Brian Zoltowski, Ph.D. '08, currently an assistant professor at Southern Methodist University, is the paper's lead author.
Identifying the structure of dCRY is "a starting point to understand the function and mechanisms of the protein," Vaidya added.
The main mechanism of the Drosophila circadian clock involves four types of proteins. Two negative proteins act as inhibitors, suppressing two others, the positive proteins. Without this suppression, the positive proteins activate genes that initiate signaling events and ultimately control the organism's diurnal rhythms. Suppression is prevented by dCRY, which goes into action in the presence of light, binding to the negative proteins and leading to their degradation. This allows the biological clock to reset and the positive proteins to function.
By identifying dCRY's structure, the researchers found that when a small molecule that is bound to dCRY, called a flavin, absorbs light, it helps an arm of the protein, called the C-terminus, to change shape, thus allowing dCRY to bind to the negative proteins. Previous studies have shown that dCRY proteins, and thus circadian rhythms, lose their function when the C-terminus is removed.
"Another intriguing aspect of cryptochromes is that they are evolutionarily related to enzymes called photolyases, which use light to repair damage to DNA caused by ultraviolet radiation" from sunlight, said Crane.
The dCRY crystal structure was found to be remarkably similar in structure to photolyases. The C-terminus, for example, lies in exactly the same pocket on the dCRY molecule as that to which damaged DNA attaches when photolyases begin repairs.
Recently, dCRY has also been implicated in magnetosensitivity -- the ability of organisms to sense magnetic fields, including that of the earth. Although more research is needed to better understand this phenomenon, the Cornell study provides a basis to begin to understand the mechanism.
Co-authors include researchers from the Laboratory of Genetics at Rockefeller University in New York. The study was funded by the National Institutes of Health.
Provided by Cornell University