Scientists develop environmentally friendly way to produce propylene oxide using silver nanoclusters

Scientists at the U.S. Department of Energy's Argonne National Laboratory have identified a new class of silver-based catalysts for the production of the industrially useful chemical propylene oxide that is both environmentally friendly and less expensive.
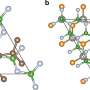
"The production of propylene oxide has a significant amount of by-products that are harmful to the environment, including chlorinated or peroxycarboxylic waste," said chemist Stefan Vajda of Argonne's Materials Science Division and Center for Nanoscale Materials. "We have identified nanoclusters of silver as a catalyst that produce this chemical with few by-products at low temperatures."
Propylene oxide is commonly used in the creation of plastics and propylene glycols for paints, household detergents and automotive brake fluids.
The study is a result of a highly collaborative team that involved five Argonne Divisions and collaborators from the Fritz-Haber-Institut in Berlin and from the University of Illinois in Chicago, including a collaboration between the experimental effort led by Stefan Vajda and the theoretical analysis led by materials chemist Larry Curtiss and nanoscientist Jeff Greeley.
Large silver particles have been used to produce propylene oxide from propylene, but have suffered from a low selectivity or low conversion to propylene oxide, creating a large amount of carbon dioxide. Vajda discovered that nanoscale clusters of silver, consisting of both three atoms as well as larger clusters of 3.5 nanometers in size, are highly active and selective catalysts for the production of propylene oxide.
Curtiss and Greeley then modeled the underlying mechanism behind why these ultrasmall nanoparticles of silver were so effective in creating propylene oxide. They discovered that the open shell electronic structure of the silver catalysts was the impetus behind the nanoclusters selectivity.
"Propylene oxide is a building block in the creation of several other industrially relevant chemicals, but the current methods of creating it are not efficient," Curtiss said.
"This is basically a holy grail reaction," remarked Greeley. "The work opens a new chapter in the field of silver as a catalyst for propene epoxidation," added Curtiss.
A paper on this work will be published in the April 9 issue of the journal Science.
Provided by Argonne National Laboratory