Atomic force microscope advance leads to new breast cancer research
Researchers who developed a high-speed form of atomic force microscopy have shown how to image the physical properties of live breast cancer cells, for the first time revealing details about how deactivation of a key protein may lead to metastasis.
The new findings also are providing evidence for the mechanisms involved in a cell's response to anti-cancer drugs, said Arvind Raman, Purdue University's Robert V. Adams Professor of Mechanical Engineering.
In atomic force microscopy (AFM), a tiny vibrating probe called a cantilever passes over a material, precisely characterizing its topography and physical properties. However, before now the procedure has been too slow to record some quickly changing biological processes in action.
"Before this advance you could see only the before and after, but not what happened in between, the dynamics of the event," Raman said. "There is evidence based on this work and our previous findings that there might be a mechanical signature to drug resistance."
Advanced models allow researchers to convert AFM data into properties about the cell's internal scaffolding, called the cortical actin cytoskeleton, including the motion of fibers called actin.
Findings are detailed in a paper appearing Monday (June 29) in the research journal Scientific Reports, the open access journal of the Nature Publishing Group. The researchers used the technique to study breast cancer cells, probing a key enzyme called spleen tyrosine kinase, or Syk.
Kinases cause phosphorylation of proteins, a biochemical process that can alter enzymes and plays a significant role in a wide range of cellular processes.
"So if you turn the kinase off, proteins will get dephosphorylated and then changes could occur," said Robert L. Geahlen, Distinguished Professor of Medicinal Chemistry at Purdue. "We were able to show the turn off of this kinase very rapidly alters the physical properties of the cell. So it's undoubtedly due to the phosphorylation events that are having immediate effects on cytoskeletal proteins."
The paper was authored by former doctoral student Alexander X. Cartagena-Rivera, now a postdoctoral fellow at the National Institutes of Health's National Institute on Deafness and Other Communication Disorders (NIDCD); Purdue postdoctoral research associate Wen-Horng Wang; Geahlen; and Raman.
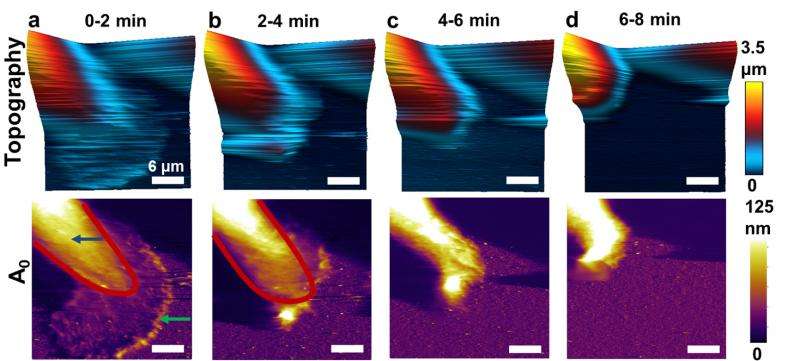
The researchers studied breast cancer cells exposed to a chemical "inhibitor" that blocks the functioning of Syk, leaving the cells free to metastasize. Because of the new higher-speed AFM, the researchers for the first time have been able to observe what happens when the inhibitor is added.
After adding the inhibitor, actin bands propagate across the cell, causing the cell to change shape.
"This takes about 10 minutes, which is quite fast compared to many biological processes," Raman said.
Discover the latest in science, tech, and space with over 100,000 subscribers who rely on Phys.org for daily insights. Sign up for our free newsletter and get updates on breakthroughs, innovations, and research that matter—daily or weekly.
The images can be taken at a speed of about 50 seconds per frame.
"Before we did this it would take roughly 15 to 20 minutes to take one frame, which is too slow to observe this transition process," he said.
Bands of actin were shown to move in a sweeping motion across the cell.
"You think of actin as a scaffolding, but it's a dynamic scaffolding," Raman said. "We can see bands of actin that are going around and changing the physical properties during the transition, which was not understood before."
When Syk is missing or deactivated, breast cancer cells undergo a process called EMT, or epithelial-mesenchymal transition, causing them to become highly motile and to undergo metastasis.
"If this kinase is in the cells, the cells cannot metastasize, so we've been trying to figure out what the mechanisms are by which you have to get rid of this kinase in order to become highly motile and metastatic," said Geahlen, who is affiliated with the Purdue Center for Cancer Research. "And that's one of the reasons we were looking at this particular type of cancer cell with this particular form of Syk in it."
One goal of the research is to correlate physical properties of cells with tumor suppression and the action of the kinase on the cell.
The advance in AFM technology was accomplished by two innovations: as the cantilever scans a cell it bends differently depending on the properties of the material being scanned. A laser measures this "deflection," and models convert the data to reveal information about the material's composition. Previous applications of AFM microscopy to study live cells provided feedback on the amplitude and frequency of the vibrating cantilever, but not the deflection. However, that approach takes too long to provide images of the quickly changing processes inside living cells. Providing feedback on the deflection instead has now been shown to increase the imaging speed 10-fold, making the method practical for studying cellular processes.
The other innovation is a technique that enables the cantilever to vibrate at two frequencies simultaneously.
"In one scan we can map the local physical properties of the cell, and we can do it fast enough that we compile maps of the changing cell," Raman said.
More information: "Fast, Multi-frequency and Quantitative Nanomechanical Mapping of Live Cells Using the Atomic Force Microscope." Scientific Reports 5, Article number: 11692 DOI: 10.1038/srep11692
Journal information: Scientific Reports
Provided by Purdue University