Animal-free reprogramming of adult cells improves safety

Human stem cells produced through genetic reprogramming are beset by safety concerns because current techniques alter the DNA of the stem cells and use material from animals to grow them. Now, A*STAR researchers have developed an efficient approach that produces safe, patient-specific human stem cells.
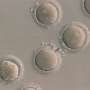
Human induced pluripotent stem cells have the potential to treat a number of diseases without the ethical issues associated with embryonic stem cells. Pluripotent stem cells can be produced from adult cells by introducing genes that reprogram them. Typically, the stem cells are grown on a layer of mouse cells in solutions (known as media) that contain animal proteins—and therefore, potentially may also carry disease. For such stem cells to be safe for use in humans, they need to be grown in 'xeno-free' conditions, which are devoid of material from other animals.
Andrew Wan and Hong Fang Lu at the A*STAR Institute of Bioengineering and Nanotechnology in Singapore and colleagues set out to develop a new xeno-free system. The researchers carried out the genetic reprogramming of cells on an artificially produced protein substrate rather than mouse cells. They also used media that contained no animal components. The result was more efficient reprogramming than seen with conventional approaches.
"A xeno-free system will eliminate the risk of disease transmission from other species, which is important for regulatory approval," explains Wan. "Yet there have been few studies on cell reprogramming under totally xeno-free conditions."
The researchers went one step further by addressing the problem of cells acquiring alterations to their DNA during reprogramming.
"Incorporation of transgenes into the genome of the cell poses another safety issue, risking unwanted genetic alterations," explains Lu. "In our work, the transgenes were introduced to initiate the reprogramming, but after this they were removed from the cell, leading to transgene-free stem cells."
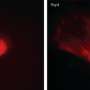
The researchers demonstrated that after genetic reprogramming and the removal of the added genes, the stem cells could still develop into different cells types. They were even able to induce them to form dopaminergic neurons, the type that degenerates in Parkinson's disease. The conditions in which the stem cells were grown mean that they are suitable for clinical use and can be derived from a patient's own cells, ensuring complete compatibility.
"Regulatory approval for clinical application of stem cells largely depends on the conditions in which the stem cells are derived," says Wan. "We present a workable protocol for the reprogramming of fibroblasts to stem cells that minimizes any potential safety risks."
More information: Lu, H. F., Chai, C., Lim, T. C., Leong, M. F., Lim, J. K. et al. A defined xeno-free and feeder-free culture system for the derivation, expansion and direct differentiation of transgene-free patient-specific induced pluripotent stem cells. Biomaterials 35, 2816–2826 (2014). dx.doi.org/10.1016/j.biomaterials.2013.12.050
Journal information: Biomaterials