Experiments show hypothesis of microtubule steering accurate
Tiny protein motors in cells can steer microtubules in the right direction through branching nerve cell structures, according to Penn State researchers who used laboratory experiments to test a model of how these cellular information highways stay organized in living cells.
"We proposed a model of how it works in vivo, in the living cell," said Melissa Rolls, associate professor of biochemistry and molecular biology. "But because of the complexity of the living cells, we couldn't tell if the model was possible."
Rolls then collaborated with William O. Hancock, professor of biomedical engineering, who was already working on the tiny kinesin motors that move materials throughout the cell, to test the model in the laboratory, in vitro.
"Kinesins are little machines that use chemical energy to generate mechanical forces sufficient to carry materials through the cell," said Hancock.
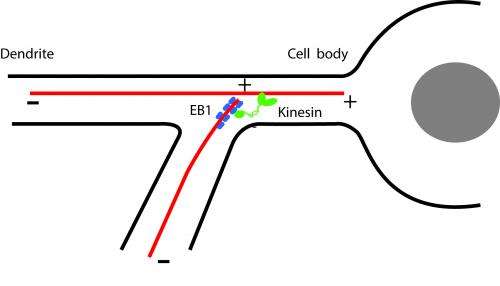
Cells produce enzymes, proteins and signaling chemicals in the center of the cell, and these materials are then moved to other cell areas by kinesin motors. Dendrites in nerves cells are very long, and motors need to transport molecules relatively long distances on microtubules that are constantly forming and dissolving within the cell. Because dendrites branch, the researchers wondered how the microtubules themselves move in the right direction.
Working with Yalei Chen, graduate student in cell and developmental biology in the Huck Institutes of the Life Sciences, the researchers found that kinesin motors can not only transport molecules along the tubules, but can redirect the ends of the tubules to enter the proper branch of the dendrite. They report their findings online today (Jan. 23) in Current Biology.
In the laboratory, the researchers grew microtubules under the microscope and used protein engineering to attach a kinesin motor to EB1—a protein that binds to the growing end of microtubules.
"One of the reasons we thought the model might not work is that the molecule EB1 grabs the plus end of the microtubule very loosely," said Rolls. "We were unsure how something so dynamic could hold the forces, but it does."
The researchers found that it is a form of crowd sourcing—while one molecule is only loosely bound and releases quickly, the microtubule's plus end is surrounded by hundreds of these molecules so the EB1 can guide the motor protein where to go. The kinesin motor walks along a stationary microtubule until it enters the branch.
In the laboratory, the combination EB1 and kinesin motor moved the microtubule ends far enough for redirection into branches.
The researchers state that "EB1 kinetics and mechanics are sufficient to bend microtubules for several seconds." They also suggest that "other kinesins also demonstrate this activity, suggesting this is a general mechanism for organizing and maintaining proper microtubule polarity in cells."
Journal information: Current Biology
Provided by Pennsylvania State University