High-powered microscopic techniques give scientists detailed view of a critical component of cellular infrastructure
The cellular interior is criss-crossed by protein-based cables known as microtubules, each formed from 13 'protofilaments' composed of the protein tubulin. Microtubules are also associated with a host of other specialized proteins that help coordinate the transport of molecular cargoes and link microtubules to intracellular structures.
A research team led by Yuko Mimori-Kiyosue from the Optical Image Analysis Unit of the RIKEN Center for Developmental Biology is involved in the study of a subset of proteins that preferentially localize near the microtubule ends and regulate their assembly and disassembly. By performing an up-close examination of two such proteins, end-binding 1 (EB1) and colonic–hepatic tumor-overexpressed gene (ch-TOG), the team have now revealed surprising new details about the organization of microtubule ends.
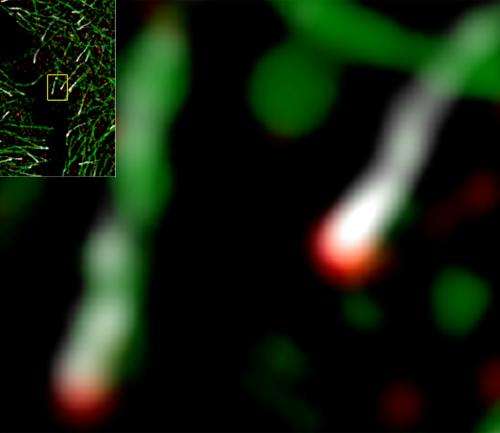
Scientists have long believed that EB1 specifically accumulates at the microtubule tip, although the detailed structure of this region has proved difficult to observe. "The dynamic configuration of microtubule ends has never been studied in living organisms, mainly due to limited resolution of microscopy techniques," explains Mimori-Kiyosue. Her team overcame these limitations through the use of an ultra-high resolution imaging strategy, and was surprised to discover that EB1 is not actually the endmost microtubule protein. EB1 typically accumulates in comet-shaped structures, and for over 90% of the EB1 comets examined, ch-TOG was situated even further along the microtubule, indicating that it instead is the endmost protein (Fig. 1).
Microtubule ends are constantly growing and shrinking as tubulin subunits are added or removed, and depletion experiments demonstrated that EB1 and ch-TOG both contribute to the maintenance of this dynamic state in an independent and complementary fashion. However, Mimori-Kiyosue's team also identified a distinct role for EB1 in attaching microtubule ends to the inner surface of the cell membrane, which it accomplishes through interaction with other specialized membrane-anchoring proteins. Having these anchor points slightly removed from the end likely prevents such interactions from interfering with tubulin addition or removal at the microtubule tip. Mimori-Kiyosue finds this novel function of EB1 particularly interesting. "Appropriate organization of the microtubule network is very important," she says, "since microtubules serve as rails for cellular trafficking, which need to be placed correctly to deliver important materials to the correct destination."
Future studies by Mimori-Kiyosue's team should further clarify the role of these proteins in microtubule maintenance. As microtubules are core components of the cell division machinery and therefore primary targets for cancer drugs, these findings could in turn facilitate the discovery of new therapeutic agents.
More information: Nakamuru, S. et al. Dissecting the nanoscale distributions and functions of microtubule-end-binding proteins EB1 and ch-TOG in interphase HeLa cells. PLoS ONE 7, e51442 (2012). dx.doi.org/10.1371/journal.pone.0051442
Journal information: PLoS ONE
Provided by RIKEN