No entry without protein recycling: Researchers discover new coherence in enzyme transport

The group of Prof. Dr. Ralf Erdmann at the Ruhr-Universität Bochum, Germany, discovered a connection of peroxisomal protein import and receptor export. In the Journal of Biological Chemistry, they disclosed that enzymes only get imported into certain cell organelles (peroxisomes) upon coupling of their import to the recycling of their transport protein (receptor).
Peroxisomes do not have their own DNA. Thus, all peroxisomal proteins are coded within the nucleus and imported into the peroxisome after their synthesis is completed. The Erdmann lab investigates this process in detail. Peroxisomes contain more than 50 various enzymes in total which e.g. decompose fatty acids and dispose hydrogen peroxide or plasmalogens, the main phospholipid of the white matter of the brain. A disruption of their function does not only cause severe metabolic disorders, it can even lead to death of newborns.
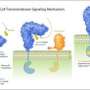
Dynamic receptors recognize and escort the enzymes destined for the peroxisome to the organelle where they attach to the membrane. Then the receptor-enzyme complex disassembles and the enzyme is transported across the peroxisomal membrane. Afterwards, the receptor is transported from the membrane back to the cytosol. This recycling is controlled by the attachment of the small protein ubiquitin to the receptor, which functions as an export signal.
The team of Prof. Dr. Ralf Erdmann studied a certain peroxisomal receptor, which consists of a targeting unit (Pex18p) and an enzyme-binding unit (Pex7p). The scientists discovered that ubiquitin modifies Pex18p in order to enable the receptor to return to the intracellular liquid. Only when the targeting unit of the receptor is exported from the membrane, the import of the cargo-loaded enzyme-binding unit takes place. This result supports the export-driven-import model, previously proposed by the Erdmann group.
More information: W. Schliebs, W. Girzalsky, R. Erdmann (2010): Peroxisomal protein import and ERAD: variations on a common theme, Nature Reviews Molecular Cell Biology, doi: 10.1038/nrm3008
Journal information: Journal of Biological Chemistry
Provided by Ruhr-University Bochum