Study offers first explanation of how cells rapidly repair and maintain structure
Researchers at Huntsman Cancer Institute (HCI) at the University of Utah have discovered that a protein, zyxin, is necessary for the maintenance and repair of the cell's cytoskeleton, or internal framework, which serves as the muscle and bone of the cell. The research has implications for cancer, as well as other diseases, since alterations in the cytoskeleton are often associated with disease. The research was published in the Sep. 14, 2010, issue of the journal Developmental Cell.
"Just like people, the cells in our bodies are exposed to all kinds of stress," says Mary Beckerle, Ph.D., the study's principal investigator and HCI executive director. "One type of stress, mechanical stress that is derived from application of physical force, is experienced by many organs such as the lung, which stretches with each breath, the heart, which is physically challenged with each beat, and the uterus, which undergoes intense contractions during labor and childbirth. We were interested in how living cells respond to such stress. In this study, we showed that mechanical stress can damage the cytoskeleton but that cells have special machinery that rapidly recognizes the damage and repairs it."
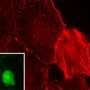
Mark Smith, Ph.D., one of the HCI researchers involved in the study explains that, "When a cell's environment changes and stress is applied, cytoskeletal bundles, called actin stress fibers, can literally begin to tear, but then are rapidly repaired. This process begins within seconds and allows the cell to retain its structure. We showed that a protein called zyxin is required for the maintenance and repair of the actin cytoskeleton." Zyxin expression is down-regulated in certain cancers and future experiments will explore whether loss of this cytoskeletal repair pathway in tumor cells is responsible for the disruption of the cytoskeleton that is common in cancer cells.
The researchers studied the process by imaging live cells that expressed fluorescently tagged cytoskeletal proteins. This allowed them to observe the mechanism whereby actin stress fibers maintain homeostasis, or balance. The repair mechanism was directly triggered by force and served to relieve mechanical stress on actin stress fibers, which in turn provided a system for rapid response to force changes in the extracellular environment.
Provided by University of Utah Health Sciences