This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Researchers discover mechanism for carbonaceous particle formation
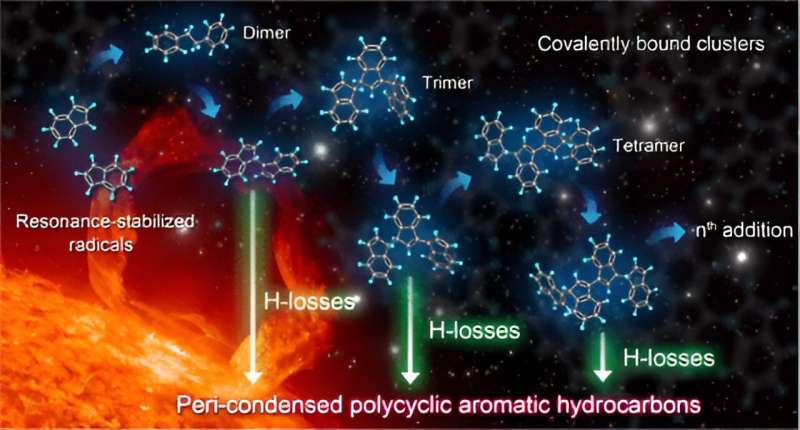
A research team led by Prof. Wang Zhandong from the University of Science and Technology of China (USTC), observed a series of covalent cluster intermediates in resonantly stabilized free radical gas-phase reactions with a synchrotron radiation vacuum ultraviolet photoionization mass spectrometry experimental platform, revealing the role of resonantly stabilized free radicals in the growth of particulate matter mass.
The study has been published in the Journal of the American Chemical Society.
Carbonaceous particles are produced in transportation, industrial emissions, and combustion. Apart from causing haze, they can induce respiratory and cardiovascular diseases if inhaled by humans.
Carbonaceous particles will also affect the performance and lifespan of engines. Therefore, there is an urgent need to reduce these particles in air. To realize that, revealing how carbonaceous particles are formed helps.
Resonantly stabilized radicals (RSR) are a special class of radicals detected in carbonaceous particles, which obtain higher stability and longer lifetime through electron delocalization. Due to limitations of experimental methods, research on the reaction mechanism of RSR mainly focuses on the reaction path, and there is no direct experimental evidence on how RSR achieves particle mass growth.
A series of regularly growing covalently bound clusters (CBCs), intermediates in the evolution from small RSRs, is observed in experiments by using in situ synchrotron radiation ultraviolet photoionization mass spectrometry (SVUV-PIMS) at the BL09U beamline of the National Synchrotron Radiation Laboratory (the undulator-based beamline was designed with an energy range of 7–124 eV to study the spectroscopy and dynamics of atoms, molecules, and clusters).
Pyrolyze halogenated radical precursors in a laminar flow reactor were chosen to conduct the experiment. Since RSR could be exclusively produced via cleavage of the C−Br bond at a high temperature in this reactor.
Combined with quantitative calculations, hydrogen atom abstraction and multiple RSR additions were found to promote the formation and rapid growth of covalent clusters. These clusters gradually dehydrogenated to produce large-sized peri-condensed condensed aromatic and polycyclic aromatic radicals and form white smoke-like carbonaceous particles.
This progressive H-losses from CBCs (PHLCBC) elucidated an important relationship among resonance-stabilized radicals, covalent clusters, and peri-condensed condensed aromatics, bridging small-sized gas-phase radical intermediates with large-sized nanocarbon particles.
This study is expected to provide important insights for multi-scale simulation of carbonaceous particulates, and guide the synthesis of carbon nanomaterials, reveal the formation mechanism of fullerene clusters in flames, and develop new coking inhibitors.
More information: Hong Wang et al, Direct Observation of Covalently Bound Clusters in Resonantly Stabilized Radical Reactions and Implications for Carbonaceous Particle Growth, Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.4c03417
Journal information: Journal of the American Chemical Society
Provided by University of Science and Technology of China