This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists probe the source of key hydrocarbons on Earth—and in space
Polycyclic aromatic hydrocarbons (PAHs) are a type of organic molecule that carry fused rings made of the chemical benzene. Scientists believe that PAHs are responsible for chemical processes that eventually lead to soot and other carbonaceous nanoparticles on Earth and around and between the stars in deep space. On Earth, PAHs form in part because of the incomplete combustion of coal, oil, and other substances and are detrimental to human health.
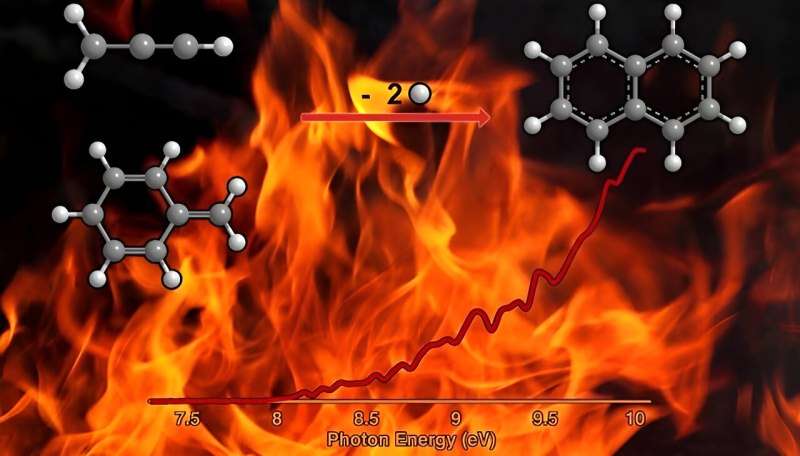
Across the universe, PAHs account for as much as 30% of all carbon, whether around stars, interstellar clouds, or planets. However, scientists do not fully understand the role of reactions involving two free radicals in how PAHs form in extreme environments. Free radicals are molecules with an unpaired electron, which is delocalized over at least three atoms. In a study published in the journal Chemical Science, researchers conducted experiments to uncover how the prototype PAH—naphthalene—can form from reactions that take place in the gas phase of matter.
The results provide fundamental knowledge on the processes that can form the simplest representative of PAHs naphthalene—a key ingredient in mothballs. The researchers found that this reaction can occur in the gas phase via the reaction of radicals that are found in combustion flames and in the space around carbon-rich stars. This provides new foundational knowledge of the chemistry and carbon balance of our galaxy.
Polycyclic aromatic hydrocarbons (PAHs) and their descendant soot particles represent unwanted byproducts in combustion processes of fossil fuel, but scientists do not have a complete understanding of the fundamental mechanisms of their formation. An isomer selective product detection reveals that the reaction of the resonantly stabilized benzyl (C7H7) and the propargyl (C3H3) radicals synthesizes the simplest representative of PAHs—the 10p Hückel aromatic naphthalene (C10H8) molecule.
The gas-phase preparation of naphthalene affords a radical new concept of the reaction of combustion relevant propargyl radicals with aromatic radicals carrying the radical center at the methylene moiety (aromatic-CH2•), which have been previously overlooked as a source of aromatics in high temperature environments.
This facile Propargyl Addition—BenzAnnulation (PABA) mechanism of propargyl radicals with other aromatic-CH2• radicals beyond benzyl could lead to higher order PAHs like anthracene and phenanthrene. This finding is a fundamental shift in the perception that PAHs are predominantly formed via the Hydrogen-Abstraction—Acetylene Addition (HACA) and Phenyl Addition DehydroCyclization (PAC) pathways in high temperature combustion settings.
This PABA mechanism offers versatile and diverse routes to three key classes of aromatic hydrocarbons: acenes (PAHs consisting of linearly fused benzene rings), phenacenes (PAHs carrying zig-zag structured benzene rings), and helicenes (ortho-condensed PAHs in which benzene rings are angularly annulated yielding helically shaped chiral molecules), thus bringing scientists closer to an understanding of the aromatic universe we live in.
More information: Chao He et al, Unconventional gas-phase preparation of the prototype polycyclic aromatic hydrocarbon naphthalene (C10H8) via the reaction of benzyl (C7H7) and propargyl (C3H3) radicals coupled with hydrogen-atom assisted isomerization, Chemical Science (2023). DOI: 10.1039/D3SC00911D
Journal information: Chemical Science
Provided by US Department of Energy