Phosphorus atoms help drive metal to form ammonia, adding insights to turning renewable energy to fuel
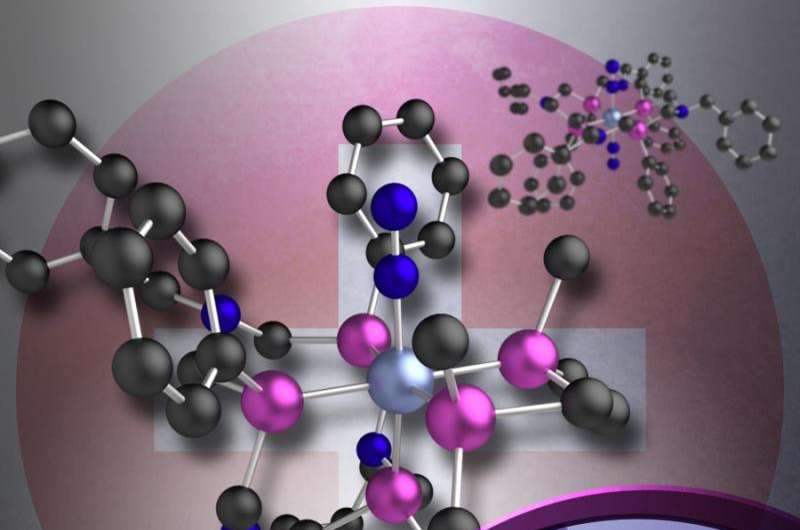
Underappreciated compared to its heavier metal counterparts, chromium failed for more than 30 years to turn nitrogen gas into ammonia, a reaction that involves breaking one tough bond and making six new ones. But scientists at the Center for Molecular Electrocatalysis thought chromium was up to the job; it just needed a little support. At the center, one of DOE's Energy Frontier Research Centers (EFRCs), the scientists created a 12-atom ring structure called a ligand that partially surrounds the metal and offers a stable environment for the metal to drive the reaction. By creating this ligand structure, the team demonstrated the importance of the environment supporting chromium. Often a key to controlling metal reactivity, the structure encircling the chromium causes the normally unreactive dinitrogen to become more reactive when it binds to the metal.
"This research required the synergy of experimental and computational efforts in an EFRC," said the study's lead Dr. Michael Mock at DOE's Pacific Northwest National Laboratory. "Studying this challenging reaction has benefited from the multiple years of funding that an EFRC enables." The EFRCs are funded by the Office of Basic Energy Sciences at DOE's Office of Science.
Producing ammonia for fertilizer consumes vast quantities of energy, an issue that this work may one day help solve. However, this study is focused on another important challenge: storing intermittent wind and solar energy. Solar panels and wind turbines produce electrons that flow along power lines to energize appliances around our homes. But, solar power levels drop when the clouds roll in. What if those electrons could be stored inside a chemical bond, as an energy-dense storage option? This study, which is complemented by two previous reports focused on understanding dinitrogen reactivity with chromium, may someday lead to the development of a system with this common metal as a hard-working catalyst.
"This research shows how important it is to move six electrons and six protons in the right order," said Dr. Roger Rousseau, who led the computational studies. "It is rather like herding cats-and very difficult cats at that."
There is a long tradition of turning dinitrogen (N2) into ammonia (NH3) using complex molecular catalysts, materials that reduce the roadblocks to make the reaction occur and aren't consumed in the process. Of the metals studied in the column known as group 6 transition metals, chromium supported by phosphorus ligands didn't work. In fact, papers from 1970 to the present day reported failures using chromium even in an environment that was thought to goad it into working.
However, Mock and his team focused on the stabilizing effect from the phosphorus atoms of a 12-membered ligand that partially surrounded the chromium metal. Every fourth atom in the ring is a phosphorus atom that forms a bond with the chromium atom. The chemical bonds formed with three phosphorus atoms of the large ring together with two additional phosphorus donor atoms of a second ligand make the chromium atom very electron rich, which then can bind the dinitrogen. Once bound, the dinitrogen triple bond is weakened by coordination to the metal.
The team showed that the correct surroundings enhance chromium's ability to bind and activate dinitrogen. In fact, the dinitrogen molecule in this case is more activated than in similar complexes with the heavier metals, molybdenum and tungsten, which have similar properties to chromium.
However, breaking the dinitrogen triple bond is still a delicate task.
The team found that managing the number of phosphorus atoms and the electron-donating ability of these atoms was crucial. The team ran the reactions with acid at -50°C so that certain intermediate products containing nitrogen-hydrogen bonds didn't fall apart. In these reactions, hydrogen ions from the acid surrounding the complex formed only a small amount of ammonia. They showed that adding acid caused the protons to favor binding with the metal, an unwanted connection. Additional optimization of the chromium complex and the conditions is required to control the formation of the desired nitrogen-hydrogen bonds.
The reaction still has secrets to reveal. The team is digging into two of them. First, how do the 12-membered rings that support the chromium form? In the experiments, the rings self-assemble around the chromium. What factors dictate that formation? Also, how can the protons be controlled to prevent them from binding to the electron-rich chromium and form additional bonds with nitrogen? Answering these questions could lead to learning how to control the reaction's environs and lead to a catalyst that is fast, efficient, and long lasting, to convert nitrogen to ammonia.
More information: Michael T. Mock et al. Protonation Studies of a Mono-Dinitrogen Complex of Chromium Supported by a 12-Membered Phosphorus Macrocycle Containing Pendant Amines, Inorganic Chemistry (2015). DOI: 10.1021/acs.inorgchem.5b00351
Journal information: Inorganic Chemistry
Provided by Pacific Northwest National Laboratory