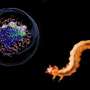
How bilogical antifreeze proteins hinder the growth of ice crystals
This week Dutch, American and Canadian researchers present a major step in understanding antifreeze proteins, the proteins that hinder the growth of ice crystals. Artificial forms of these proteins are interesting for a whole range of applications – from de-icing spray and road salt to improved preservation of frozen food and organs. The team, led by TU/e researcher Ilja Voets, will publish its findings this week in the leading journal PNAS on how we need a different type of antifreeze protein than previously thought for most applications.
For nature it's no problem at all. Arctic fish, snow scorpionflies and a whole range of other creatures have amazing proteins that ensure their blood does not freeze in the cold environment in which they live. These antifreeze proteins, of which there are tens of varieties, attach to small ice crystals thereby hindering their growth. So while ice may form in the blood, the crystals are so small that the blood is not deprived of its functions.
Low-calorie ice cream
The big scientific challenge is to duplicate these proteins and get a grip on how they work. For instance, to help improve the quality of frozen food or organs or to boost the resilience of plants against the freezing cold. They could also be used in a spray to de-ice car windscreens or aircraft wings, or as an additive to road salt (less salt needed) or ice cream (fewer calories).
Two kinds of activity
Roughly speaking, antifreeze proteins work in two ways. On the one hand, they reduce the temperature whereby ice crystals begin to grow rapidly (the scientific term being 'thermal hysteresis', or TH). On the other hand, they combat so-call recrystallization, the process by which, in simple terms, small ice crystals cluster into larger chunks (scientific term: 'ice recrystallization inhibition', or IRI). However, the relationship between these two activities of antifreeze proteins has long been unclear.
Activity
TU/e researcher Ilja Voets and her team of Dutch, American and Canadian researchers now reveal that there is no clear relationship between these activities and that there are also significant differences per protein. This also means that it is not so easy, as had long been thought, to determine how 'active' a protein is – an important consideration in its suitability in applications. "Sometimes the TH activity is important but more often than not it is the IRI activity that appears to be the determining factor," says Voets.
Tunnel vision
This conflicts with the prevailing view. "A kind of tunnel vision had emerged for proteins that were labeled as the 'most active'," Voets explains. "But these proteins by no means turn out to be the most suitable candidate for applications, according to our study. The type of protein we should be targeting depends on the specific application. In other words, we need to customize."
More information: Blocking rapid ice crystal growth through nonbasal plane adsorption of antifreeze proteins. DOI: 10.1073/pnas.1524109113
Journal information: Proceedings of the National Academy of Sciences
Provided by Eindhoven University of Technology