October 1, 2015 feature
First observation made of quantum-tunneling diffusion of hydrogen atoms on ice
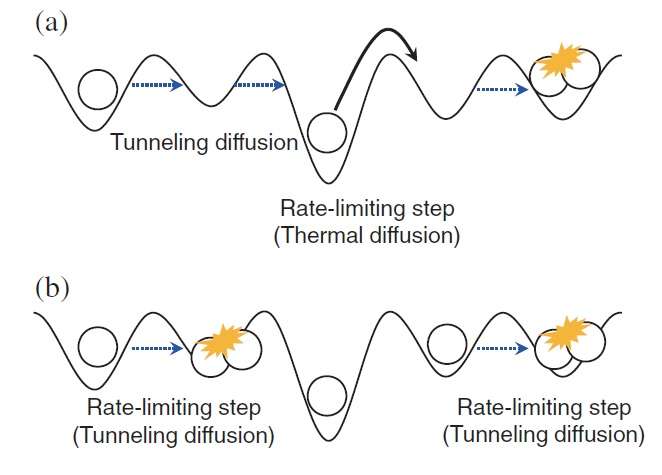
(Phys.org)—As long as the temperature is above absolute zero, gas molecules are always in constant random motion. They may diffuse—or spread out—through three-dimensional space or, in a process called "surface diffusion," along the two-dimensional surface of a solid. The most well-known mechanism to explain surface diffusion is a classical mechanism called thermal hopping, in which gas molecules jump from one adsorption site (the place where the gas molecules attach to the surface) to another at a temperature-dependent rate. So far, thermal hopping is the only mechanism known to explain the surface diffusion of hydrogen atoms on the surface of ice.
Now in a new study, scientists from Hokkaido University in Japan have reported the first evidence of quantum tunneling as a mechanism for the surface diffusion of hydrogen atoms on the surface of ice, although quantum tunneling has previously been observed for hydrogen atoms on the surface of some metals. In quantum tunneling, the hydrogen atoms can move through barriers that they otherwise could not pass through using only classical mechanisms, such as thermal hopping.
"This is the first observation of hydrogen-atom tunneling diffusion on an ice surface," coauthor Naoki Watanabe, Professor at Hokkaido University, told Phys.org.
The new finding means that surface diffusion in this situation may work differently than previously thought. This may have implications for understanding the formation of H2 molecules in interstellar clouds, as the same hydrogen-on-ice surface diffusion process occurs on the surface of cosmic ice just before two hydrogen atoms combine to form an H2 molecule.
Since quantum tunneling cannot be observed directly, the scientists had to detect its presence using a creative approach and some high-tech, laser-based analytical tools. In the previous studies on metal surfaces, quantum-tunneling diffusion was detected by taking advantage of the fact that, unlike thermal hopping, quantum tunneling is temperature-independent. Unfortunately, in the ice regime that the scientists investigated here, the temperatures are so low (below 20 K) that the temperature window in which diffusion occurs is too narrow to measure temperature dependence.
Instead, the scientists took advantage of an isotope effect. Based on previous experimental data and calculations on thermal hopping and the kinetic energies of gases, the scientists predicted that, at 10 K, hydrogen atoms (which have one proton and one electron) should diffuse at a rate that is 4.5 times faster than that of deuterium atoms (which have one proton, one electron, and one neutron). If the rate difference between the two isotopes were to significantly differ from 4.5 in experiments on ice, then the difference could not be explained by thermal hopping, and would be clear evidence of quantum-tunneling diffusion.
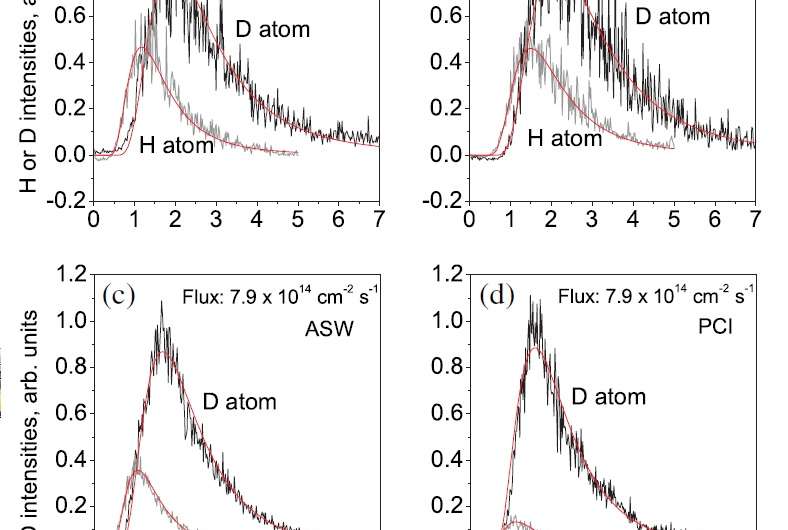
As it turned out, the new experimental results showed that, at 10 K, hydrogen atoms diffuse 100 times faster than deuterium ions on a highly ordered type of ice called polycrystalline ice. The researchers concluded that this larger-than-expected kinetic isotope effect can only be explained by quantum tunneling.
The researchers also found that, on a less-ordered type of ice called amorphous solid water, the rate difference is about 16, which is only somewhat higher than expected. The scientists believe that this small increase suggests that quantum tunneling may play a role in the surface diffusion on this type of ice, but thermal hopping is likely still the dominant mechanism. The reason why quantum tunneling may be suppressed on amorphous solid water may have to do with its nonperiodic structure, whereas polycrystalline ice has a periodic structure.
Another interesting part of the study is the way that the researchers determined the rates—not by direct measurement, but by using two laser techniques called photostimulated desorption (PSD) and resonance-enhanced multiphoton ionization (REMPI). The PSD laser beam first desorbs/detaches the adsorbed gas atoms on the ice surface, and then the REMPI laser ionizes the desorbed atoms. The researchers then measured the ion intensities, which are proportional to the number of desorbed atoms. By comparing the ion intensities of the desorbed hydrogen and deuterium atoms, the scientists could determine the ratio of their concentrations, which in turn could be used to find the ratio of their diffusion rates.
As the scientists explained, the results could lead to a better understanding of the astrochemical H2 formation process, and they plan to pursue this area more in the future.
"The diffusion mechanism—whether tunneling or thermal hopping—strongly depends on the morphology of the ice surface," Watanabe said. "Cosmic ice has various ranges of morphology. This work demonstrates how H atoms on cosmic ice diffuse toward H2 formation. The H atoms alternate between tunneling diffusion and thermal hopping until encountering a reaction partner of another H atom. In the future, we would like to continue with similar experiments on heavier atoms such as oxygen and radicals, which also exist on cosmic ice and contribute to the formation of molecules on the cosmic ice."
More information: K. Kuwahata, et al. "Signatures of Quantum-Tunneling Diffusion of Hydrogen Atoms on Water Ice at 10 K." Physical Review Letters. DOI: 10.1103/PhysRevLett.115.133201
Journal information: Physical Review Letters
© 2015 Phys.org