Key blood pressure drug seen in startling new detail
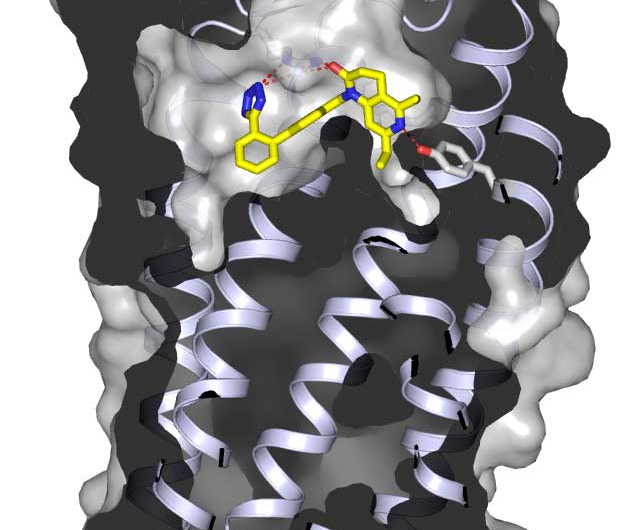
A new Arizona State University research study has revealed the fine details of how an experimental drug works to regulate blood pressure, paving the way to the development of better drugs.
The ASU team's interdisciplinary work, led by Petra Fromme of the Biodesign Institute, may one day help scientists better control blood pressure irregularities with a new class of drugs that could limit harmful side effects.
The new research focuses on a type of drug known as an angiotensin II receptor blocker, or ARB. Used by millions of people each year, ARBs are designed to block a receptor so it cannot bind with angiotensin II, a hormone that constricts blood vessels to cause an increase in blood pressure.
"Uncovering the structure of the angiotensin receptor is a real breakthrough in the development of better drugs to regulate blood pressure," Fromme says. It has been unraveled by a new technique called femtosecond crystallography, pioneered by researchers at ASU and their collaborators.
The technique for uncovering these atomic scale structures uses a jet of tiny crystals that are exposed to super-strong X-ray pulses. These powerful bursts of light destroy any solid material but are so short that the structure of the receptor can be discovered before the molecule is destroyed. The structure reveals – in atomic detail – how the drugs block the receptor.
"Many ARBs have been developed, but the interaction between the drug and the receptor has been unknown at the atomic level," said Vadim Cherezov, a chemistry professor at the University of Southern California (USC) who led the experiment in collaboration with nine other institutions at SLAC's Linac Coherent Light Source (LCLS) X-ray laser, a DOE Office of Science User Facility.
Solving the structure of the joined ARB and receptor brought researchers a number of surprises. "We have shown that all of the previous molecular models – the best guesses for how receptor and drug fit together – were wrong in many important details," Cherezov said.
Results of the new study – the first to examine the detailed structure of a drug-receptor complex through high-speed X-ray crystallography – are detailed in the April 23 advanced online edition of Cell.
Under pressure
Given the importance of anti-hypertensive drugs, researchers have sought to uncover agents or mechanisms that will more actively and efficiently control blood pressure, streamline therapy and improve results.

One third of U.S. adults suffer from high blood pressure or hypertension and take prescription medication to treat it. Known as a "silent killer" because the condition often produces no outward symptoms, hypertension is a major risk factor for heart disease and stroke, the leading causes of death in the U.S.
ARBs are a multibillion-dollar industry, with fewer side effects than other hypertension medications. But because their effectiveness at higher doses may be limited, they are often used in combination with other drugs, complicating treatment when patients fail to follow the complete regimen.
In the vast majority of cases, hypertension occurs in primary form, meaning that its underlying causes are unknown. In secondary hypertension, the condition arises as a result of a known affliction, including chronic kidney disease, arterial narrowing or a preexisting endocrine disorder.
Left unchecked, severely elevated blood pressure can lead to a "hypertensive crisis," a condition carrying significant risk of complications. These may include visual deterioration due to retinopathy, breathlessness accompanying heart failure or acute kidney failure. Generally, a hypertensive crisis occurs when some trigger causes already elevated blood pressure to sharply spike.
Crystal clear
The new study examined nanoscale crystals of an experimental ARB coupled to an angiotensin II receptor. The study of such medically important molecules is carried out using brief pulses of intense X-ray light. The tool of choice, a high-speed laser capable of taking vivid snapshots of biological material, uses X-rays a billion times more powerful than conventional light sources.
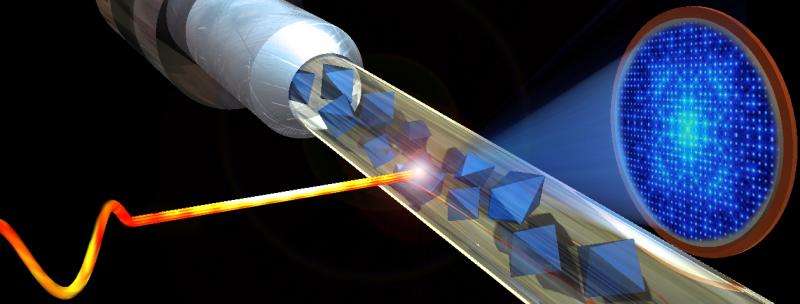
Like an inkjet printer, a fine spray of tiny crystals of the ARB-receptor pair is injected onto the path of the X-ray beam, which instantly vaporizes the sample.
Crystalized samples are imaged on the scale of femtoseconds. A femtosecond is 0.3 "light micrometers," a timespan so brief that in 1 femtosecond, a light ray would cross a distance barely the size of a virus. The concentrated X-ray beam vaporizes the sample, but the pulse is so short that the delicate structure is captured before the molecule is destroyed.
Using femtosecond X-ray crystallography, the researchers were able to precisely determine the crystal structure of angiotensin II type1 receptor at a remarkable resolution, down to 2.9 angstroms (one ten-billionth of a meter – the width of a single hydrogen atom).
Contributions from ASU researchers included the crystallization of samples and biophysical characterization of the drug-receptor constructs, data collection and evaluation, as well as development of the devices that deliver the stream of nanocrystals.
The work is based on a team effort of ASU faculty Petra Fromme, Wei Liu and Uwe Weierstall with their teams of researchers and students, including: Nadia Zatsepin, researcher in the Department of Physics; graduate students Chelsie Conrad and Jesse Coe from the Department of Chemistry and Biochemistry; and Daniel James, Dingjie Wang and Garrett Nelson from the Department of Physics.
In the future, the researchers hope to study the receptor in combination with other drug compounds to fill in even more details and accelerate the design of new drugs to modulate blood pressure and improve human health.
"A further exciting avenue would be to construct a molecular movie of the function of the receptor and the changes induced by the drug binding," said Fromme. "By obtaining a movie of the dynamics of the receptor and the drug binding, we can eventually design structure-based drugs that block the receptor in action, which might be more efficient and specific, thereby minimizing side effects for the patients."
More information: Cell, www.cell.com/cell/abstract/S0092-8674(15)00428-6
Journal information: Cell
Provided by Arizona State University