Study sheds light on how malaria parasites grow exponentially
A University of South Florida College of Public Health professor and his team of researchers have become the first to uncover part of the mysterious process by which malaria-related parasites spread at explosive and deadly rates inside humans and other animals.
As drug-resistant malaria threatens to become a major public health crisis, the findings could potentially lead to a powerful new treatment for malaria-caused illnesses that kill more than 600,000 people a year.
In a study published online March 3 in the high-impact journal PLOS Biology, the USF researchers and their colleagues at the University of Georgia discovered how these ancient parasites manage to replicate their chromosomes up to thousands of times before spinning off into daughter cells with perfect similitude – all the while avoiding cell death.
"How these parasites preserve fidelity in this seemingly chaotic process is one of the great mysteries of this pathogen family," said USF Health's Michael White, PhD, a professor in the Department of Global Health who partnered on the study with fellow USF researcher Elena Suvorova, PhD, in the USF Departments of Molecular Medicine & Global Health and the Florida Center for Drug Discovery and Innovation, as well as with two researchers from the University of Georgia.
In studying the malaria-relative Toxoplasma gondii, the team found an explanation for that puzzle.
To understand it, consider that malaria-related parasites are professional multipliers, unlike plant and animal species and single-cell organisms like yeast – where chromosomes get one shot at replication or else the cell dies or turns into cancer, Dr. White explained.
With malaria-related parasites, once transmitted into an animal or human, they can hide out in a single cell in many different tissues replicating silently tens, hundreds or even thousands of times before the host's immune system can detect that they are there.
Then with the stealth of a Trojan horse, they burst forth as "daughter cells," which are unleashed in massive quantities in waves, like a small army into the host's system – quickly overwhelming a patient's immune response, Dr. White explained.
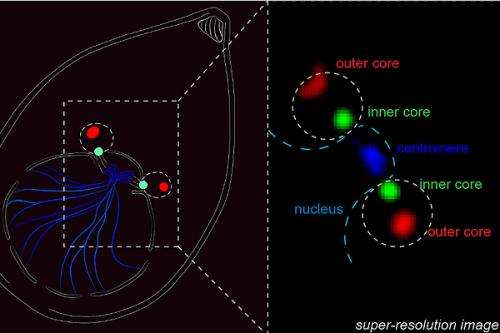
What the study found was that the Toxoplasma parasites pull this off thanks to a "modified 'control room' called the centrosome that imposes order on the replication chaos," Dr. White said. "Unlike the comparatively simple centrosome present in human cells, the parasite 'control room' has two distinct operating machines; one machine controls chromosome copying, while the other machine regulates when to form daughter cell bodies. Working together, but with independent responsibilities, parasite centrosome machines can dictate the scale and timing of pathogen replication."
This groundbreaking understanding and novel discovery of the centrosome's function leads to a critical conclusion: disruption of the centrosome machines – like cutting the cables between two computer systems – kills the parasite, Dr. White said.
Breaking any part of the highly efficient but highly fragile replication functions shuts everything down.
"They are literally Humpty Dumpty," he added. "If they break, they can't be put back together."
With these findings and the new knowledge of the parasites' vulnerabilities, Dr. White and his fellow researchers will delve into drug development.
That process could take anywhere from four to 10 years of further research and clinical trials before a new drug is on the market, he said. The length of time depends on whether the researchers hit upon effective application of prior-FDA approved cancer-related drugs or develop a new treatment from scratch.
Whatever treatment they develop, Dr. White stressed that it will be used in conjunction with other types of drug therapies.
Currently drugs used to treat malaria go after the pathogens' metabolism, while the new research will seek to undermine the parasite's foundation in enough of the spreading cells in order to allow the human immune system to fight back and not become overwhelmed, Dr. White said.
A major challenge today in parts of the world is the lack of access to drug treatments at all or until it is too late and the patient succumbs to malaria-related illnesses and brain hemorrhaging.
Because of the parasite's high-adaptability, current drug treatments are constantly susceptible to the development of drug resistance, Dr. White said.
A potential global health crisis is unfolding as drug-resistant malaria continues to move across Burma, reaching the Indian border, according to British newspaper The Independent, commenting on a recent study in the journal Lancet. Doctors fear it will continue to spread and enter Africa, home to 90 percent of the world's malaria cases.
Malaria caused about 207 million cases and 627,000 deaths in 2012, according to the Centers for Disease Control and Prevention. About 3.2 billion people, or half the world's population, are at risk of malaria, according to the World Health Organization.
Dr. White said that this study, which he called the first for a USF Health laboratory in publishing original research in PLOS Biology, will help get more potential treatments in the pipeline.
"The more we understand their vulnerability," he said of the parasites, "the better chance we can keep that pipeline full."
More information: "A Novel Bipartite Centrosome Coordinates the Apicoplexan Cell Cycle," Elena S. Suvorova, Maria Francia, Boris Striepen and Michael W. White, PLOS Biology, March 3, 2015; DOI: 10.1371/journal.pbio.1002093
Journal information: PLoS Biology , The Lancet
Provided by University of South Florida

















